Three different types of reactions with aqueous solutions are as follows:
- 1. Precipitation Reactions These reactions take place when two aqueous reactants, one solid and one liquid, react to form an insoluble product which is called a precipitate. For example when lead nitrate mixes with potassium iodide as shown in the following chemical reaction: ...
- 2. Acid-base Reaction As we know acid contains positive hydrogen ions. ...
- 3. Oxidation-Reduction Reactions
What type of reactions occur in aqueous solutions of water?
Water has many unique properties and are present in abundance on Earth due to which reactions in aqueous solutions occur frequently. The three main types of reactions that occur in aqueous solutions are precipitation reactions, acid-base reactions, and redox reactions. The reactions that occur in aqueous solutions mostly involve ions.
Which of the following is an example of aqueous solution?
An example of an aqueous solution is sodium chloride i.e. common salt dissolved in water. Three different types of reactions with aqueous solutions are as follows: 1. Precipitation Reactions These reactions take place when two aqueous reactants, one solid and one liquid, react to form an insoluble product which is called a precipitate.
What happens when water is the solvent for a reaction?
When water is the solvent for a reaction, the reaction is said to occur in aqueous solution, which is denoted by the abbreviation (aq) following the name of a chemical species in a reaction. Three important types of reactions in water are precipitation, acid-base, and oxidation-reduction reactions.
What are the 3 types of chemical reactions?
1.6: What is Energy? Many chemical reactions occur in aqueous solutions. These reactions fall into 3 main categories: precipitation, acid–base, and oxidation–reduction. In a typical precipitation reaction, dissolved cations and anions come together to form an insoluble ionic compound that precipitates from the solution.

What type of reaction occurs when ions are present in aqueous solution?
Precipitation reactionsPrecipitation reactions occur when cations and anions in aqueous solution combine to form an insoluble ionic solid called a precipitate.
What happens when aqueous solutions react?
A precipitate is formed when ions in solution react with each other to form an insoluble product. Solubility rules help to identify the precipitate that has been formed. A number of tests can be used to identify whether certain anions (chlorides, bromides, iodides, carbonates, sulphates) are present in a solution.
When reactions occur in aqueous solutions what common types of products are produced?
The three common types of produced by reactions that occur in aqueous solutions are precipitates, water or gas.
Is an aqueous solution a chemical reaction?
When chemical reactions occur between species in an aqueous solution, the reactions are usually double replacement reactions. In such reactions, the cation from one reactant takes the place for the cation in the other reactant. Hence it is typically forming an ionic bond.
How does aqueous solution occur?
An aqueous solution is one in which the solvent is liquid water. That is, solute (dissolved) ions and molecules are surrounded by water molecules and incorporated into the network of bonds within the water. The dissolved species then spread throughout the water.
What are aqueous reactions?
Aqueous reactions are reactions that take place in water. To understand them, it is important to understand how compounds behave in water. Some compounds are electrolytes- they dissociate into separate ions in water. However, not all electrolytes behave the same way.
Why do chemical reactions occur more readily in aqueous solutions?
Answer. Ionic compounds in solution react faster than molecular compounds. This is because Ionic compounds break apart to form free ions. Therefore, there are no bonds to break so the reaction is fast.
How do you know if a reaction is aqueous or solid?
If a compound dissolves in water then you get an aqueous solution formed. If it does not dissolve in water then you get a solid precipitate.
What happens when aqueous solutions mix?
Aqueous solutions of ionic compounds are comprised of the ions making up the compound dissociated in water. These solutions are represented in chemical equations in the form: AB(aq) where A is the cation and B is the anion. When two aqueous solutions are mixed, the ions interact to form products.
What happens when you add two aqueous solutions?
The solid that forms when two aqueous solutions are combined is called a precipitate .
What happens to acid and base in aqueous solution?
Hence, acids and bases mix in water to dissociate and form hydronium and hydroxide ions respectively. Additional information: Acids and bases react together to form salts. This is called a neutralization reaction, and can yield various important salts, like sodium chloride, as HCl+NaOH→NaCl+H2O .
Do aqueous solutions react faster?
Aqueous ions tend to react faster than species in other states of matter: Solid lead(II) nitrate will react with solid potassium iodide, but the reaction is really, really slow. That's because the ionic bonding in each reactant is strong and the ions in each compound are hard to separate from each other.
What is the aqueous state of a substance?
As the name implies, aqueous state refers to the state of a substance that has been dissolved in water. When scientists write chemical reactions, they use the symbol (aq) to designate when a substance is dissolved in an aqueous solution. If we want to convert a solid salt crystal to the aqueous state, we would simply dissolve it in water. Once dissolved, the atoms that made up the salt crystal (sodium and chlorine) are now in the aqueous state. That is, they are now solutes within a water-based solution. But it is important to distinguish between the aqueous state and the liquid state because the former is more specific. For example, a salt crystal could be converted to a liquid state without being dissolved in water. This could be done by heating it beyond its melting point to create a molten liquid salt. Other liquids and solutions that are not in the aqueous state include substances dissolved in organic solvents such as alcohols (methanol), aromatics (toluene), ketones (acetone), and hydrocarbons.
What is the process of dissociating a polar solid into a water solution?
The separation of the sodium and chloride ions from the solid and their subsequent dispersion into the water solvent is called dissociation. Substances composed of charged particles such as sodium chloride (NaCl), magnesium chloride, calcium fluoride, iron sulfide etc. are called polar solids or polar compounds. Water is an excellent solvent for polar compounds because the unequal charge distribution of the water molecule allows it to pull ions off of solid materials by electrostatic attraction effectively. For example, the negative charge of the oxygen atom on the water molecules attracts the positively charged sodium ion in salt. This attractive force exerted by the water molecule weakens the bond that holds the sodium and chloride together, thus facilitating their dissociation into the water solvent. This process creates a sodium chloride aqueous solution otherwise known as saltwater.
How to tell if a solution is aqueous or non-aqueous?
For example, non-aqueous organic solutions (alcohol, acetone, toluene, benzene, etc.) commonly contain volatile compounds that can be easily detected by scent. Many of these organic solutions also evaporate more readily than aqueous solutions. Another important way to know if a solution is aqueous is to determine what type of materials dissolve in it. Water readily dissolves substances that break into charged particles (ions) when they go into solution (e.g., salts such as sodium chloride). Other types of solvents, such as alcohol or cooking oils, do not readily dissolve salts.
What is the symbol for a chemical reaction?
As mentioned above, when scientists write chemical reactions, they use the symbol (aq) to designate when a substance is dissolved in an aqueous solution. For example, when salt (sodium chloride) dissolves in water, the sodium and chloride atoms are dispersed as charged particles called ions. The sodium ion has a positive charge, and the chloride ion has a negative charge. Positively charged particles in solution are called cations while negatively charged particles are called anions.
What happens when ions are dissociated?
As shown in the diagram below, when the ions are dissociated, they become surrounded by water molecules. The negatively charged part of the water molecules loosely links to the sodium ions, and the positively charged parts surround the chloride ions. This process is called solvation, and it plays an important role in keeping the ions in solution, thus preventing them from reattaching and forming new sodium chloride crystals.
What is an example of an aqueous fluid?
An important biological example of an aqueous fluid is the water-based, transparent liquid that gives human eyes the rigidity needed to allow sight. This liquid is called aqueous humor. It is vital in the field of ophthalmology because of its critical role in aligning the cornea and lens of the human eye and its role in supplying nutrients and oxygen to eye tissues. So, without the solvent properties of water, human sight, as we know it, would not be possible.
How are the properties of aqueous solutions determined?
The properties of aqueous solutions are determined by the molecular structure and characteristics of the water molecule. The diagram shows that a single water molecule is V-shaped and consists of a single oxygen atom bonded to two hydrogen atoms. The nature of the bonds between the oxygen and hydrogen atoms leads to an unequal sharing of electrons. The oxygen atom becomes negatively charged because it attracts electrons (negatively charged particles) away from the hydrogen atoms, which become positively charged.
Reactions In Aqueous Solutions Definition
Reactions in aqueous solutions are the reactions that occur in the solution that uses water to dissolve or break down a substance.
Overview of Reactions In Aqueous Solutions
Many reactions in living systems, mostly chemical or biological reactions, take place in water. Aqueous solution is a solution in which the solvent is water and thus, it can be said that these reactions take place in aqueous solution.
Ions in Aqueous Solution
The oxygen atom in the water molecule has a slightly negative charge and the hydrogen atoms have slightly positive charges. Thus, the water molecule is polar in nature. So substances that are ionic in nature dissolve in water to form ions in the aqueous solution.
Types of Reactions in Aqueous Solution
Precipitation reactions, acid-base reactions, and oxidation-reduction are the three main types of reactions. While the precipitation and acid-base reactions involve exchange of ions, the Redox reactions involve the transfer of electrons from one molecule to another molecule or ion.
How to write a balanced equation?
Two important principles apply when writing balanced equations for reactions between species in a solution: 1 The balanced equation only includes the species that participate in forming products. For example, in the reaction between AgNO 3 and NaCl, the NO 3- and Na + ions were not involved in the precipitation reaction and were not included in the balanced equation. 2 The total charge must be the same on both sides of a balanced equation. Note that the total charge can be zero or non-zero, as long as it is the same on both the reactants and products sides of the equation.
What is an example of oxidation reduction?
In an oxidation-reduction or redox reaction, there is an exchange of electrons between two reactants. The species that loses electrons is said to be oxidized. The species that gains electrons are said to be reduced. An example of a redox reaction occurs between hydrochloric acid and zinc metal, where the Zn atoms lose electrons ...
What is the reaction between HCl and NaOH?
Acid-Base Reactions. For example, when hydrochloric acid, HCl, and sodium hydroxide, NaOH, are mixed, the H + react s with the OH - to form water: HCl acts as an acid by donating H + ions or protons and NaOH acts as a base, furnishing OH - ions.
What are the three types of reactions that occur in water?
Three important types of reactions in water are precipitation, acid-base , and oxidation-reduction reactions.
What is the reaction of an anion and a cation?
In a precipitation reaction , an anion and a cation contact each other and an insoluble ionic compound precipitate out of solution. For example, when aqueous solutions of silver nitrate, AgNO 3, and salt, NaCl, are mixed, the Ag + and Cl - combine to yield a white precipitate of silver chloride, AgCl:
Which species are not included in the balanced equation?
The balanced equation only includes the species that participate in forming products. For example, in the reaction between AgNO 3 and NaCl, the NO 3- and Na + ions were not involved in the precipitation reaction and were not included in the balanced equation.
Why are weak electrolytes poor conductors?
It is because of that strong electrolytes completely dissociate into ions in water , whereas weak electrolytes incompletely dissociate. When chemical reactions occur between species in an aqueous solution, the reactions are usually double ...
What happens when a chemical reaction occurs between species in an aqueous solution?
When chemical reactions occur between species in an aqueous solution, the reactions are usually double replacement reactions. In such reactions, the cation from one reactant takes the place for the cation in the other reactant. Hence it is typically forming an ionic bond. Reactions in aqueous solution may result in the products which are soluble in ...
What is the name of the reaction that takes place when two aqueous reactants, one solid and one liquid?
1. Precipitation Reactions. These reactions take place when two aqueous reactants, one solid and one liquid, react to form an insoluble product which is called a precipitate. For example when lead nitrate mixes with potassium iodide as shown in the following chemical reaction: Pb (NO 3) 2 + 2KI => PbI 2 + 2KNO 3.
Why is water an aqueous solution?
Due to this reaction in aqueous solutions occur frequently. Water molecules are containing two hydrogen atoms bonded to a single oxygen atom. Many substances can dissolve in water and hence give an aqueous solution.
What are some examples of solutions that are not aqueous?
Some examples of solutions that are not aqueous solutions include any liquid that does not contain water.
What is the meaning of the word "aqueous"?
The word aqueous is also applicable to describe a solution or mixture in which water is the solvent. A substance will form an aqueous solution or not, it depends on the nature of its chemical bonds. When a substance dissolves in water, this is denoted by writing (aq) after its chemical name.
What happens to the acid and base reaction?
Due to acid and base reaction, a neutralization reaction occurs.
What happens in a precipitation reaction?
In a typical precipitation reaction, dissolved cations and anions come together to form an insoluble ionic compound that precipitates from the solution. For example, the reaction between aqueous potassium chloride and aqueous silver nitrate leads to the precipitation of insoluble silver chloride.
What is the difference between a precipitation and an oxidation reaction?
In an acid-base reaction, an acid reacts with a base, and the two neutralize each other, producing salt and water. An oxidation–reduction reaction involve s the transfer of electrons between reacting species. The reactant that loses electrons is said to be oxidized, and the reactant that gains electrons is said to be reduced.
What is the difference between water-soluble and ionic solids?
When a chemical reaction occurs in an aqueous solution, water-soluble molecular solids dissolve as intact molecules, whereas water-soluble ionic solids exist as separated ions. Ionic solids that are water-insoluble remain undissolved.
What are the three types of chemical reactions that occur in water?
For this reason, many chemical reactions take place in water. Such reactions are called aqueous reactions. The three most common types of aqueous reactions are precipitation, acid-base, and oxidation-reduction.
How long is the Jove trial?
Please click here to activate your free 12-hour trial. If you do not wish to begin your trial now, you can log back into JoVE at any time to begin.
Why is the balanced equation called the molecular equation?
The balanced equation is called the molecular equation because the complete neutral formulas of the compounds are written as if they existed as molecules or whole units in solution.
What are the three main categories of chemical reactions?
Many chemical reactions occur in aqueous solutions. These reactions fall into 3 main categories: precipitation, acid–base, and oxidation–reduction.
What is acid rain?
Acid rain refers to the deposition of acidic components in rain, snow and dew. Acid rain occurs when sulphur dioxide and nitrogen oxides are emitted into the atmosphere, undergo chemical transformations and are absorbed by water droplets in clouds. The droplets then fall to earth as rain, snow, mist, dry dust, hail, or sleet.
What is the water that passes through a media bed?
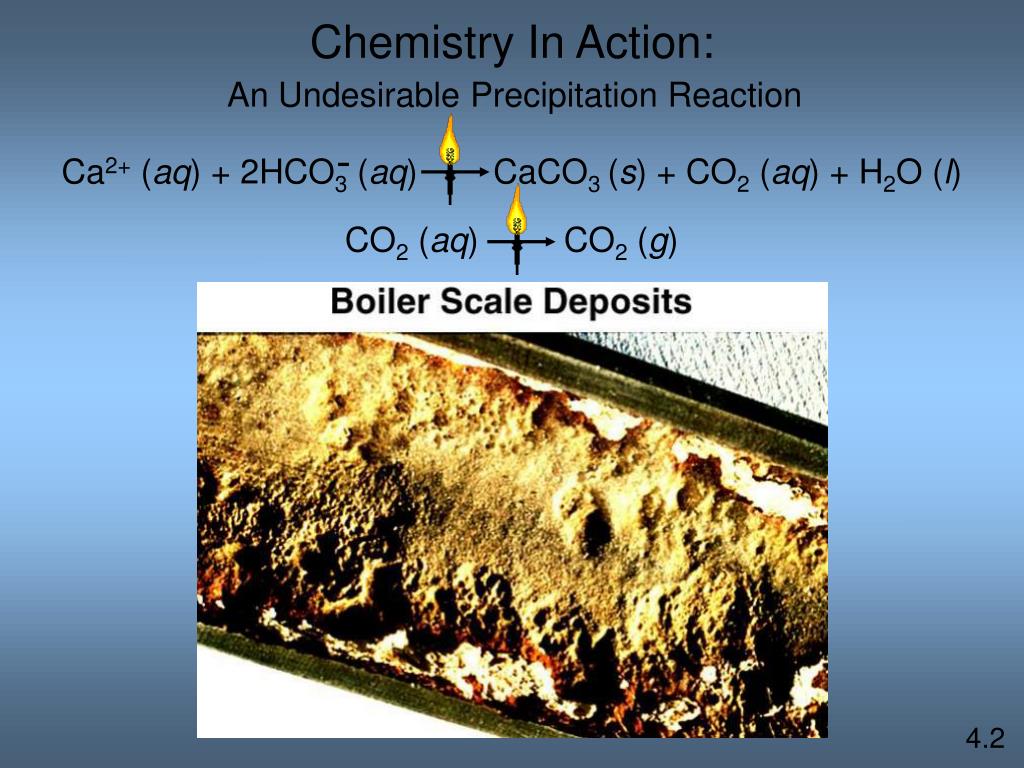
Hard water passes through a media bed, usually made of resin beads that are supersaturated with sodium. As the water passes through the beads, the hardness minerals (e.g. calcium and magnesium) attach themselves to the beads. The sodium that was originally on the beads is released into the water.
How does acid rain affect the environment?
Acid rain can have a very damaging effect on the environment. In rivers, dams and lakes, increased acidity can mean that some species of animals and plants will not survive. Acid rain can also degrade soil minerals, producing metal ions that are washed into water systems. Some of these ions may be toxic e.g. Al3 +.
What is soft water?
Water that has a low mineral content is known as soft water. If water has a high mineral content, it usually contains high levels of metal ions, mainly calcium ( Ca) and magnesium ( Mg ). The calcium enters the water from either CaCO3 (limestone or chalk) or from mineral deposits of CaSO4.
How does carbon dioxide affect the soil?
This increases the acidity of the soil and affects the chemical balance of lakes and streams. Carbon dioxide Carbon dioxide reacts with water in the atmosphere to form carbonic acid ( H2CO3 ). CO2 + H2O → H2CO3. The carbonic acid dissociates to form hydrogen and hydrogen carbonate ions.
What are some examples of ions that can be lost during exercise?
It is also important to realise that our bodies can lose ions such as Na +, K +, Ca2 +, Mg2 +, and Cl -, for example when we sweat during exercise. Sports drinks such as Lucozade and Powerade are designed to replace these lost ions so that the body's normal functioning is not affected. Dissociation in water.
How to find the dissolution of sodium chloride?
Sodium chloride dissolves in water. The dissolution of sodium chloride can be represented by the following equation: NaCl (s) → Na + (aq) + Cl - (aq) The symbols s (solid), l (liquid), g (gas) and aq (material is dissolved in water) are written after the chemical formula to show the state or phase of the material.