
What is meant by cation and anion?
What is meant by cation and anion?
- The electronic configuration of many ions is that of the closest noble gas to them in the periodic table.
- An anion is an ion that has gained one or more electrons, acquiring a negative charge.
- A cation is an ion that has lost one or more electrons, gaining a positive charge.
What is an example of a cation and anion?
Ions: Points to Remember
- Atom is neutral, having equal number of protons and electrons whereas ion is electrically charged by removing or adding electrons from a neutral atom.
- Ion possesses an electrical charge. ...
- It is basically an atom or a group of atoms having a negative/positive charge.
What are the cations and anions in the periodic table?
What are examples of anions?
- bromide Br –
- chloride Cl –
- fluoride F –
- iodide I –
- nitride N 3–
- oxide O 2–
- sulfide S 2–
Are the cations all metals or non metals?
Metals usually have just one electron in the outer orbital, and it is removed easily, forming the cation. This kind of single electron oxidation is just a broad generalization : it does not apply to ALL metals. The principle nonmetals have outer orbitals that are missing one electron.
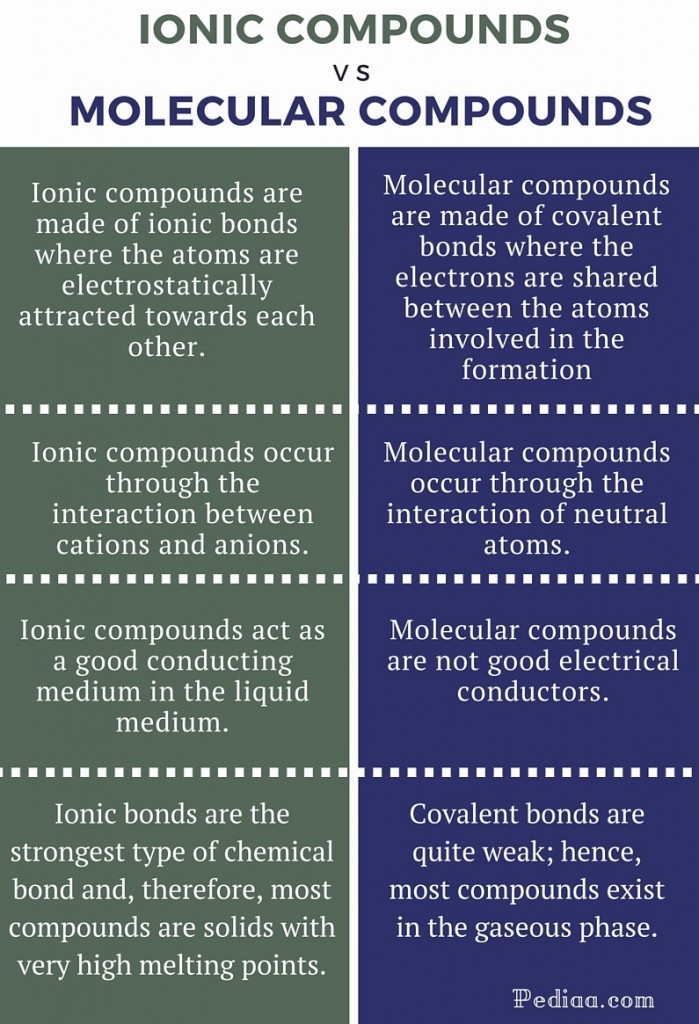
What is the difference between anions and cations?
An anion is a molecule or a group of molecules with one or more negative electric charges. Cations have one or more positive charges attached to th...
What is the use of cation and anion?
Demineralized water is produced using cation-anion ion exchangers. It is critical that both positively and negatively charged ions are eliminated d...
Give example of cations
The two most frequent resins used in the ion-exchange process are anion and cation resins. Negatively charged ions are attracted to anion resins, w...
What is an example of anion?
Cl- is an example of an anion. A Cl- atom is a chlorine atom that has gained one electron and hence has a negative charge of -1 since it has a full...
Is oxygen a cation or anion?
Oxygen is neither a cation or an anion because it has no charge. Oxygen makes up around 20% of the air, and it’s a highly reactive gas. There are v...
1. List some uses of the cation.
Cations are the ones that are positively charged and have more positive particles. There are many uses of cations in our daily life few are listed...
2. Explain the cation and anion exchange resin.
In the ion-exchange process, the most commonly used two resins are cation resin and anion resins. In this ion-exchange process, the positive partic...
3. Is carbon a cation or anion?
Elements are not generally considered as cations or anions. Few may be considered as one when they undergo chemical reactions. The ones that are ge...
4. List some uses of the anion.
Anions are the ones that are negatively charged and have more negative particles. There are many uses of anions in our daily life few are listed be...
5. Why is soil fertility cation exchange capacity considered important?
In soil fertility, cation exchange is considered a vital reaction for checking the basicity and acidity and correcting the same of the soil. It is...
What is the difference between an anion and a cation?
An anion may be defined as an atom or molecule that is negatively charged. A cation may be defined as an atom or molecule that is positively charged. Anions and cations are both ions. They have an opposite electrical charge, therefore they get attracted to each other.
How do cations and anions differ?
They have an opposite electrical charge, therefore they get attracted to each other. Cation repels other cation whereas anion repels another anion. The number of protons is more than the number of electrons in a cation whereas the number of electrons is more than the number of protons in an anion.
What are ions and cations?
Cations and anions introduction: Ions which are a part of the science subject Chemistry forms from atoms and electrons that have either gained or lost their weight by the removal or adding of one or more valence electrons which would create either positive or a negative charge.
What are ions with negative charges called?
The ions with a negative charge are called anions and the ones with a positive charge are called cations. Since both of them have charges of opposing qualities, they get attracted to one another and thereby forming an ionic bond between them.
Which anions have a net negative charge?
Therefore, they possess a net negative charge. Some examples of anions are Iodide (I – ), chlorine (Cl – ), hydroxide (OH – ). When sodium a cation is depicted as Na +, the plus charge indicator shows that it has one electron less than the total number of protons.
Do cations lose electrons?
They lose one or more than one electron and do not lose any protons. Therefore, they possess a net positive charge. Some examples of cations are Calcium (Ca 2+ ), Potassium (K + ), hydrogen (H + ).
Quick summary
Cations are positively-charged ions (atoms or groups of atoms that have more protons than electrons due to having lost one or more electrons). Anions are negatively-charged ions (meaning they have more electrons than protons due to having gained one or more electrons). Cations are also called positive ions, and anions are also called negative ions.
What is a cation?
A cation is an ion with positive charge, which means it has more protons (positively-charged particles) than electrons (negatively-charged particles). Cations are formed when an atom loses one or more electrons: the loss of the negatively-charged electron (s) results in an overall positive charge.
What is an anion?
An anion is an ion with negative charge, meaning it has more electrons than protons. Anions are formed when an atom gains one or more electrons: the gain of the negatively-charged electron (s) results in an overall negative charge.
What is the difference between cations and anions?
The difference between cations and anions is, of course, their charge. But this difference in charge has an impact on how cations and anions behave and react.
How to use cation vs. anion
Remember, cations are positive ions—they are positively charged because they have lost one or more electrons and therefore have more protons than electrons.
Looking for more explanation?
Explore science topics like protons, electrons, and neutrons with the help of a Dictionary Academy Tutor™. Tutors aren’t just the people who help you conquer subjects you’re struggling with, they can also offer study tips, strategies, and advice from an educator’s perspective.
How are cations and anions different?
The first difference is that anions possess negative electrical charges while the cations have positive electrical charges. Secondly, during electrolysis, anions move to the positive electrode which is referred to as the anode. However, the cations migrate to the negative electrode called cathode. Another difference between the two ions is that anions have more electrons than protons while the cations have more protons than electrons. Then there’s the difference that comes due to the etymology of the words. Anion comes from the Greek word “ano” which means “up.” On the contrary, the cation derives its meaning from a Greek word “kata” which means “down.” Finally, anions are non-metals while the cations are metals.
What is the purpose of anions and cations?
Application of the Concept of Anions and Cations. Ions conduct electricity through liquids or solutions. Hence the anions and cations are responsible for the phenomenon of the flow of electric current in a dry cell. The current will always flow towards the direction of the positive charge. On the other hand, it moves to the opposite direction ...
How many electrons do alkali metals lose?
The alkali metals lose one electron, alkaline metals lose two electrons, and the aluminum and other group three elements lose three electrons. On the contrary, halogens gain one electron; the group 6A elements gain two electrons whereas the group 5A elements gain three electrons. Either one or several atoms and molecules may form an anion.
Where does the word "anion" come from?
Anion comes from the Greek word “ano” which means “up.”. On the contrary, the cation derives its meaning from a Greek word “kata” which means “down.”. Finally, anions are non-metals while the cations are metals.
Is an anion a monoatomic or polyatomic?
Either one or several atoms and molecules may form an anion. If the anion is formed by one atom, it is called a monoatomic anion. However, when it is formed by two or more atoms or molecules, then it is a polyatomic anion.
Which is larger, anions or cations?
Cations are smaller in size than anions. Anions are generally larger in size than cations. 7. Cations gain electrons and convert into neutral atoms or molecules. Anions generally lose electrons and convert into neutral atoms or molecules. 8. Cations form electrostatic or ionic bonds with anions to form ionic compounds.
What is the difference between an atom and an ion?
Ion can be defined as an atom or molecule that has a net electrical charge. In an atom the number of electrons and protons is equal so they possess zero charge or neutral in nature. While in ions the number of protons are equal but number of electrons are unequal. So, they possess either positive or negative charge.
What are ions with positive charge?
Those ions which possess positive charge are called cations. Na+, Al+3, Ce+3 etc. are examples of cations . When an atom loses its electron/s then it gains a positive charge due to less number of electrons than protons in its nucleus. Then this positively charged species is called cation.
What are negative charged ions?
Those ions which possess negative charge are called anions. O-2 , CN-, OH- , Cl- etc. are examples of anions. When an atom gains electron/s to get stability, then it gains a negative charge due to more number of electrons than protons in its nucleus. Then this negatively charged species is called anion.
What is a negatively charged particle called?
Then this negatively charged species is called anion. The term was coined by Faraday and Whewell. It is taken from the Greek word ἄνω ἰόv (anion) which means ‘going up’. S.No. Cation. Anion. 1. An ion or a charged particle which has a positive charge is called cation.
What are some examples of ions?
Na+, O-2, NH4+, OH- etc. are examples of ions. Ions which are a group of elements or atoms that are covalently bonded but carry either positive or negative charge are called polyatomic ions. CN-, OH-, NH4+, NO3-, CO3-2 etc. are examples of polyatomic ions.
Who invented the term "ion"?
The term ‘ion’ was 1st used by English physicist and chemist Michael Faraday in 1834 after a suggestion by English Polymath, Scientist, William Whewell. Michael Faraday used this term for those species which were moving one electrode to another through aqueous medium.
What is the difference between anion and cation resin?
The difference between anion and cation resins is that one is positively charged ( anion) and the other is negatively charged (cation). Negatively charged ions are attracted to positively charged anion resins vs. cation resins, which attract positive ions with their negative charge.
What is a cation resin?
Cation and anion exchange resins are common and essential components in modern industrial water treatment systems. Source water often contains many dissolved minerals that must be removed lest they wreak havoc on sensitive industrial systems.
What is weak acid cation resin?
Weak acid cation resins can also be used for demineralization and dealkalization. In most water treatment cases; weak acid cation resins are used to remove divalent ions associated with alkalinity. Weak acid cation resins work best when water has a hardness and alkalinity ratio of 1:1 or higher. Contact WaterProfessionals® for help in specifying ...
What is ion exchange resin made of?
Ion exchange resins are made up of a highly porous, polymeric material that is acid, base and water insoluble. The tiny beads that make up these resins are derived from hydrocarbon and are about ½ millimeter in diameter. There are two main types of resins used for ion exchange: anion and cation. Within these two types, there are four main ...
What are the physical and chemical properties of resin?
cation resin, and which will help to determine the best resin for your needs, such as: The size of the resin beads. The amount of water the resin can hold. The amount of ions the resin can filter out before needing to be regenerated.
Can you use strong acid cation resin?
Strong acid cation resins can be used for dealkalization (most commonly in hydrogen form) or for split stream dealkalization. However, both methods of dealkalization require the use of hazardous acids to regenerate resin beds, as well as the use of a degassifier to remove carbon-dioxide created during the treatment process.
What is the difference between a cation and an anion quizlet?
What is the difference between a cation and an anion? … An anion is a negatively charged ion that has gained more electrons than protons and added them to it’s energy levels. A cation is a positively charged ion that has lost electrons from it’s energy levels. It also has more protons than neutrons.
What is difference between cation and anion Class 9?
Textbook solution. Remember, anions are negatively charged while cations are positively charged. Cations are attracted towards the cathode end of an electric field. Anions are attracted towards the anode end of an electric field.
Cations and anions introduction
Ions which are a part of the science subject Chemistry forms from atoms and electrons that have either gained or lost their weight by the removal or adding of one or more valence electrons which would create either positive or a negative charge.
Difference Between Anions and Cations
Cations are positively charged ions. They are formed when a metal loses its electrons. They lose one or more than one electron and do not lose any protons. Therefore, they possess a net positive charge. Some examples of cations are Calcium (Ca
What is anion exchange resin?
The two most frequent resins used in the ion-exchange process are anion and cation resins. Negatively charged ions are attracted to anion resins, while positively charged ions are attracted to cation resins.
What is an example of anion?
Cl- is an example of an anion. A Cl- atom is a chlorine atom that has gained one electron and hence has a negative charge of -1 since it has a full outer shell.
What is an ion?
An ion is a general term which indicate either a positively charged or negatively charged species. Ionic or electrovalent compounds undergo dissociation in solution to form cations and anions. The no.of ions formed depends on the charge on cation and anion. 2.7K views.
Is an ion positively or negatively charged?
Doda Ahmed. Answered 4 years ago. ion is a charged molecule or atom… anion is a negatively charged molecule or atom due to gain of electron therefore number of -ve charges are more than +ve charges so molecule or atom becomes negatively charged.. cation is a positively charged molecule or atom due to loss of electron therefore number ...
Is an ion a cation or an ion?
A cation is a positively charged atom or molecule, an anion is a negatively charged atom or molecule while an ion is simply a charged atom or molecule. So cations and anions are types of ions.

Quick Summary
What Is A cation?
- A cation is an ion with positive charge, which means it has more protons (positively-charged particles) than electrons (negatively-charged particles). Cationsare formed when an atom loses one or more electrons: the loss of the negatively-charged electron(s) results in an overall positive charge.
What Is An anion?
- An anion is an ion with negative charge, meaning it has more electrons than protons. Anionsare formed when an atom gains one or more electrons: the gain of the negatively-charged electron(s) results in an overall negative charge. You won’t have any negative feelings for this discussion on the differences between commonly confused hormones like serotonin and dopamine.
What Is The Difference Between Cations and anions?
- The difference between cations and anions is, of course, their charge. But this difference in charge has an impact on how cations and anionsbehave and react. One example is in the process of electrolysis, which involves an electric current passing through a material and producing a chemical reaction. During electrolysis, the positively charged cations are attracted to a negativel…
How to Use Cation vs. Anion
- Remember, cations are positiveions—they are positively charged because they have lost one or more electrons and therefore have more protons than electrons. Anions are negativeions—they are negatively charged because they have gained one or more electrons and therefore have more electrons than protons. When you’re taking your chemistry test, just remember that cats are alw…
What Determines The Loss Or Gain of electrons?
Differences Between A Cation and An Anion
- The first difference is that anions possess negative electrical charges while the cations have positive electrical charges. Secondly, during electrolysis, anions move to the positive electrode which is referred to as the anode. However, the cations migrate to the negative electrode called cathode. Another difference between the two ions is that ani...
Application of The Concept of Anions and Cations
- Ions conduct electricity through liquids or solutions. Hence the anions and cations are responsible for the phenomenon of the flow of electric current in a dry cell. The current will always flow towards the direction of the positive charge. On the other hand, it moves to the opposite direction of the flow of the negative charge carriers. In a dry cell, electricity flows out of the cathode and fl…