
BONE. The majority of the total body stores of calcium and phosphorus are located in bone in the form of hydroxyapatite [Ca 10 (PO 4) 6 (OH) 2 ]. Trabecular (cancellous) bone is located predominately in the epiphyses of the long bones, and cortical (compact) bone is in the shafts of long bones.
Full Answer
What is the main storage site of calcium in the body?
Bones are the main storage site of calcium in the body. Your body cannot make calcium. The body only gets the calcium it needs through the food you eat, or from supplements. If you do not get enough calcium in your diet, or if your body does not absorb enough calcium, your bones can get weak or will not grow properly.
Do bones need calcium and phosphorus?
Bones Need Both Calcium and Phosphorus. Both calcium and phosphorus are found naturally in dairy products, but most calcium supplements and calcium-fortified foods and beverages don't contain phosphorus. More than half of all bone is made from phosphate, and small amounts are also used in the body to maintain tissues and fluids.
What is phosphorus in the body?
Phosphorus is a mineral. Like calcium, it is very common in the earth—and in your own cells. Phosphorus is stored in your bones and teeth, and is part of each cell membrane. In your blood, phosphorus plays a vital role in your use of energy.
Where does amorphous calcium phosphate occur?
Amorphous calcium phosphate comprises the remainder; it occurs in areas of active bone formation and matures through several intermediate stages to hydroxyapatite.
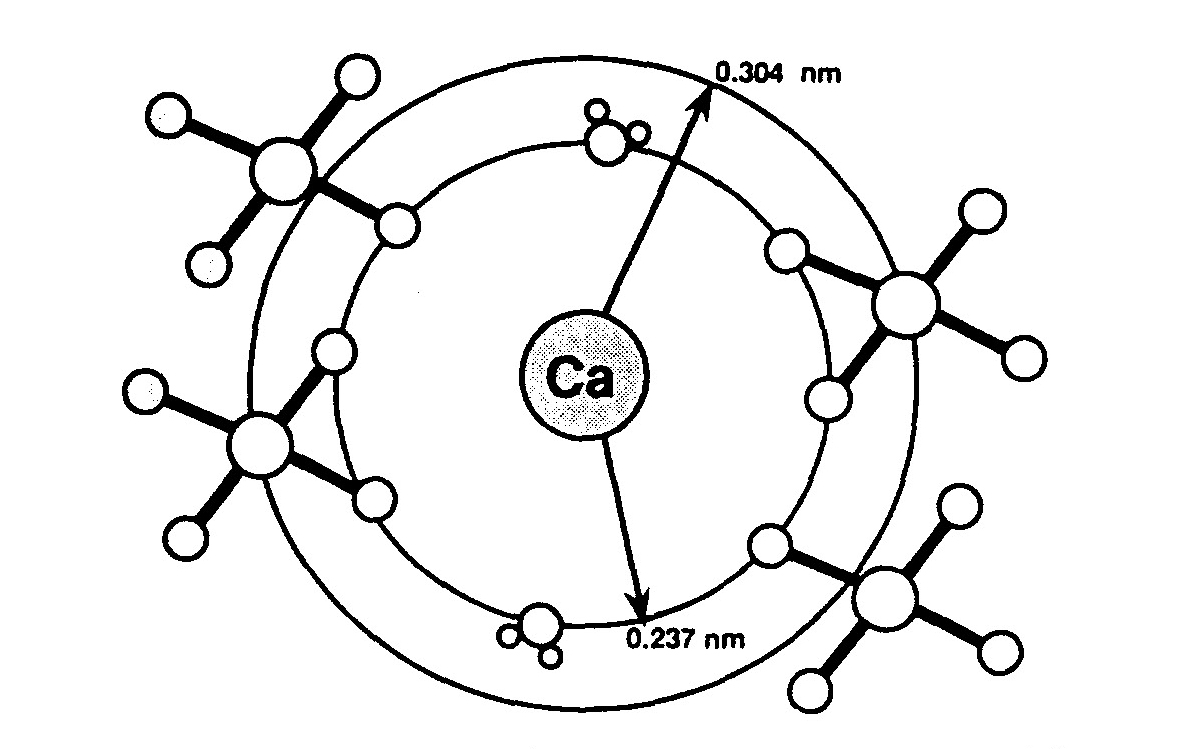
Where is calcium and phosphorus stored?
bonesYour body also needs calcium (as well as phosphorus) to make healthy bones. Bones are the main storage site of calcium in the body.
Where is calcium stored in the bone?
Calcium as a nutrient is most commonly associated with the formation and metabolism of bone. Over 99 percent of total body calcium is found as calcium hydroxyapatite (Ca10[PO4]6[OH]2) in bones and teeth, where it provides hard tissue with its strength.
How do bones store calcium and phosphorus?
It then travels in the blood, with some eventually stored with another element, phosphorus, in bone crystals, which increase the strength of bone. Calcium remains there until it is required - for example, when the levels of calcium in the blood fall - when it is released.
Where are calcium and phosphate ions stored in bone?
As with calcium, the majority of body phosphate (approximately 85%) is present in the mineral phase of bone. The remainder of body phosphate is present in a variety of inorganic and organic compounds distributed within both intracellular and extracellular compartments.
Is calcium stored in bone marrow?
Bones have many functions. They support the body structurally, protect our vital organs, and allow us to move. Also, they provide an environment for bone marrow, where the blood cells are created, and they act as a storage area for minerals, particularly calcium.
What 2 minerals are stored in bones?
Bone composition and structure The major minerals found in bone are calcium and phosphorus in the form of an insoluble salt called hydroxyapatite [chemical formula: (Ca)10(PO4)6(OH)2].
Do bones store phosphorus?
In human adult body, about 90% of total phosphorus is stored in bone as hydroxyapatite (calcium-phosphate) crystals (2). Most of the remaining phosphorus is distributed in soft tissues, and phosphate is predominantly an intracellular ion.
Where do bones store minerals?
The central cavity of long bones is filled with marrow. The red marrow is responsible for forming red and white blood cells. It stores and releases minerals and fat. The mineral component of bone, in addition to providing hardness to bone, provides a mineral reservoir that can be tapped as needed.
Is phosphate found in bone tissue?
The majority of phosphate is present in bone and teeth (85 %), with the remainder distributed between other tissues (14 %) and extracellular fluid (1 %).
Where is calcium stored in the muscle?
the sarcoplasmic reticulumCalcium ions at rest are stored in the sarcoplasmic reticulum (SR) from which they are rapidly released upon the depolarisation of the sarcolemmal and transverse (T-) tubular membranes of the muscle cell.
What gland controls calcium and phosphorus?
The parathyroid glands lie behind the thyroid. They produce parathyroid hormone, which plays a role in regulating the body's blood level of calcium and phosphorus. Hyperparathyroidism is when your parathyroid glands create high amounts of parathyroid hormone in the bloodstream.
What is calcium phosphate in bone?
Calcium phosphate (CaP) is the main mineral found in human bone and teeth and is considered as a highly biocompatible inorganic biomaterial (LeGeros, 2008). From: Nanostructures for Cancer Therapy, 2017.
Why is calcium stored in bones?
Calcium is a mineral your body needs to build and maintain strong bones and to carry out many important functions. Calcium is the most abundant mineral in the body. Almost all calcium in the body is stored in bones and teeth, giving them structure and hardness.
Where is calcium stored in the muscle?
the sarcoplasmic reticulumCalcium ions at rest are stored in the sarcoplasmic reticulum (SR) from which they are rapidly released upon the depolarisation of the sarcolemmal and transverse (T-) tubular membranes of the muscle cell.
Where is calcium stored in the muscle quizlet?
Calcium ions are stored in the sarcoplasmic reticulum.
Where is calcium found?
Calcium, a metallic element, is fifth in abundance in the earth's crust, of which it forms more than 3%. It is an essential constituent of leaves, bones, teeth, and shells. Never found in nature uncombined, it occurs abundantly as limestone, gypsum, and fluorite.
What is the role of Ca and Pi in bone resorption?
Pi plays a role in the maturation of osteocytes, the most abundant cells in bone. Osteocytes are implicated in bone mineralization and systemic Pi homeostasis.
What are the duets of calcium and phosphate?
Calcium and phosphate: a duet of ions playing for bone health. The acquisition and maintenance of bone mass and strength are influenced by environmental factors, including physical activity and nutrition.
What are the factors that influence bone mass?
The acquisition and maintenance of bone mass and strength are influenced by environmental factors, including physical activity and nutrition. Among micronutrients, calcium (Ca) and inorganic (i) phosphate (P) are the two main constituents of hydroxyapatite, the bone mineral that strengthens the mechanical resistance of the organic matrix. Bone contains about 99% and 80% of the body's entire supply of Ca and P, respectively. The Ca/P mass ratio in bone is 2.2, which is similar to that measured in human milk. The initial step of Ca-Pi crystal nucleation takes place within matrix vesicles that bud from the plasma membrane of osteogenic cells and migrate into the extracellular skeletal compartment. They are endowed with a transport system that accumulates Pi inside the matrix vesicles, followed by the influx of Ca ions. This process leads to the formation of hydroxyapatite crystal and its subsequent association with the organic matrix collagen fibrils. In addition to this structural role, both Ca and Pi positively influence the activity of bone-forming and bone-resorbing cells. Pi plays a role in the maturation of osteocytes, the most abundant cells in bone. Osteocytes are implicated in bone mineralization and systemic Pi homeostasis. They produce fibroblast growth factor-23, a hormonal regulator of renal Pi reabsorption and 1,25-dihydroxy vitamin D production. This relationship is in keeping with the concept proposed several decades ago of a bone-kidney link in Pi homeostasis. In contrast to their tight association in bone formation and resorption, Ca and Pi renal reabsorption processes are independent from each other, driven by distinct molecular machineries. The distinct renal control is related to the different extraskeletal functions that Ca and Pi play in cellular metabolism. At both the renal and the intestinal levels, interactions of Ca and Pi have been documented that have important implications in the acquisition and maintenance of bone health, as well as in osteoporosis management. In the kidney, increased Pi intake enhances Ca reabsorption and Ca balance. During growth and adulthood, administration of Ca-Pi in a ratio close to that of dairy products leads to positive effects on bone health. In contrast, when separately ingested as pharmaceutical salt supplements, thus inducing large differences between Ca and Pi concentrations in the intestinal lumen, they might have adverse effects on bone health. In osteoporotic patients treated with anabolic agents, a Ca-Pi supplement appears to be preferable to carbonate or citrate Ca salt. In conclusion, Ca and Pi constitute a key duo for appropriate bone mineral acquisition and maintenance throughout life. Outside the skeleton, their essential but distinct physiological functions are controlled by specific transporters and hormonal systems that also serve to secure the appropriate supply of Ca and Pi for bone health. Key teaching points: Bone contains about 99% and 80% of the body's supply of Ca and P, respectively, as hydroxyapatite and has a Ca/P mass ratio of about 2.2, close to that measured in human milk. The first step of Ca-Pi crystal nucleation takes place within matrix vesicles that bud from the plasma membrane of osteogenic cells. In addition to their structural role, both Ca and Pi influence bone-forming and bone-resorbing cells. There is a bone-kidney link in Pi homeostasis in which fibroblast growth factor-23, a molecule produced by osteocytes, appears to play a pivotal role. In contrast to their tight association during bone formation and resorption, both intestinal and renal Ca and Pi processes are independent of each other. Observational and interventional studies suggest that Ca-Pi salt or dairy products can exert positive effects on bone acquisition and maintenance.
Where does Ca-Pi crystal nucleation take place?
The first step of Ca-Pi crystal nucleation takes place within matrix vesicles that bud from the plasma membrane of osteogenic cells. In addition to their structural role, both Ca and Pi influence bone-forming and bone-resorbing cells.
Where is the WHO Collaborating Center for Osteoporosis Prevention located?
1Division of Bone Diseases, WHO Collaborating Center for Osteoporosis Prevention, University Hospitals and Faculty of Medicine, Geneva, Switzerland. [email protected]
Is Ca-Pi salt good for osteoporosis?
In osteoporotic patients treated with anabolic agents, a Ca-Pi supplement appears to be preferable to carbonate or citrate Ca salt. In conclusion, Ca and Pi constitute a key duo for appropriate bone mineral acquisition and maintenance throughout life.
Is Ca-Pi salt or dairy good for bone?
Observational and interventional studies suggest that Ca-Pi salt or dairy products can exert positive effects on bone acquisition and maintenance.
Why do we need calcium out of our bones?
In many adults, hormonal signals have to take some calcium out of the bones every day to keep blood calcium levels normal. This contributes to bone loss. Because of this, as you age, your body still needs calcium to keep your bones dense and strong.
How does the body get calcium?
The body only gets the calcium it needs through the food you eat, or from supplements. If you do not get enough calcium in your diet, or if your body does not absorb enough calcium, your bones can get weak or will not grow properly. Your skeleton (bones) are a living organ.
How long does it take for bones to regenerate?
Your skeleton (bones) are a living organ. Bones are constantly being remodeled with old bone being resorbed and new bone being formed. It takes about 10 years for all the bone in your body to be renewed. That is why paying attention to bone health is important in adults and not just in growing children. Bone density refers to how much calcium and ...
How to prevent bone loss?
Remaining fit and active can also protect bones and keep them stronger. Avoiding smoking also protects bones and keeps them stronger.
What is the mineral that helps muscles, nerves, and cells work normally?
The mineral calcium helps your muscles, nerves, and cells work normally. Your body also needs calcium (as well as phosphorus) to make healthy bones. Bones are the main storage site of calcium in the body. Your body cannot make calcium .
What foods help bones stay strong?
Avoiding smoking also protects bones and keeps them stronger. High-calcium foods include: Milk. Cheese. Ice cream. Leafy green vegetables, such as spinach and collard greens.
When is bone density highest?
Bone density refers to how much calcium and other minerals are present in a section of your bone. Bone density is highest between ages 25 and 35. It goes down as you get older.
What are the sources of phosphates?
Other dietary sources of phosphates include eggs, cereals, and meats.
Can calcium build bones?
March 20, 2002 -- Calcium alone can't build strong bones and tissues. New research shows calcium needs phosphorus to maximize its bone-strengthening benefits, and taking a lot of calcium supplements without enough phosphorus could be a waste of money. Researchers say it's the first time the two elements have been shown to be co-dependent ...
Does calcium interfere with phosphorus absorption?
But the study, presented at a meeting of osteoporosis experts, found that taking large amounts of calcium from supplements can interfere with phosphorus absorption.
Is calcium a co-dependent element?
Researchers say it's the first time the two elements have been shown to be co-dependent for bone health. Both calcium and phosphorus are found naturally in dairy products, but most calcium supplements and calcium-fortified foods and beverages don't contain phosphorus.
Does calcium supplement help bone mass?
If this happens, the calcium won't do much good because bone material consists of both calcium and phosphorus," says Heaney. Researchers say their study shows both calcium and phosphorus are needed to support any increase in bone mass, and a calcium supplement that contains phosphorus would be preferable to one that provides calcium alone.
Which organs regulate calcium and phosphate?
In this chapter, we review calcium and phosphate homeostasis including the critical organs involved (skeleton, parathyroids, GI tract, kidneys etc.) as well as the hormones (PTH, vitamin D, FGF23, calcitonin) that regulate calcium and phosphate.
How is calcium absorbed into the skeleton?
Calcium reaches the skeleton by being absorbed from the diet in the GI tract.
What are the major regulators of cellular calcium transport?
For the enterocyte, vitamin D enhances the movement of calcium into the cell through its stimulation of calbindin synthesis (6). For kidney tubules, PTH and FGF23 are the key regulators for the transport of calcium and phosphate (1,5,9). For bone, PTH and CT are the major regulators of cellular calcium and phosphate transport, while vitamin D provides appropriate concentrations of these minerals through it’s GI and perhaps renal actions (1-3).
What are the three minerals that are involved in bone and mineral metabolism?
The major regulation of bone and bone mineral metabolism results from the interactions of four hormones – parathyroid hormone (PTH), vitamin D (VD), fibroblast growth factor 23 (FGF23) and calcitonin (CT) – at three target organs – bone, kidneys, and GI tract – to regulate three bone minerals – calcium, magnesium, and phosphorus. Other hormones also play a role, and skin is a participating organ system (Table 1). The deviations from this normal regulatory scheme that occur in disease states can be evaluated, diagnosed, and, in most cases, effectively managed by the clinician when considered in the light of bone and mineral homeostasis (1-3).
How is extracellular calcium regulated?
Serum and extracellular calcium concentrations in mammals are closely regulated within a narrow physiologic range that is optimal for the many cellular functions. (1,2). More specifically, it is the ionized component of serum calcium that is closely regulated, as it subserves the physiological functions of this divalent cation (Table 3). Ambient calcium is so close to its saturation point in the respect to phosphates that deviations in concentrations of either can cause precipitation. Intracellular calcium, which serves as second messenger in many signal transduction pathways, is also tightly controlled, but at concentrations several orders of magnitude lower than extracellular calcium. Extraskeletal calcium accounts for only 1% of the total body calcium, as calcium is primarily sequestered in bone (Table 4-6). The average diet contains about 1 gm of calcium, but there are great variations. About 500 mg undergoes net absorption from the diet, and the unabsorbed and secreted components appear in the stool (Table 6-9). Approximately 10,000 mg/day is filtered at the glomerulus and most is reabsorbed by the renal tubules, with only a few hundred milligrams appearing in urine each day (Tables 10 and 11). The skeleton turns over about 250 mg/day of calcium, but there is wide variation. This turnover is attributed to a labile calcium pool near bone surfaces, but it is difficult to give anatomical assignment to either labile or non-labile calcium compartments. The turnover is mediated by bone-forming osteoblasts and bone-resorbing osteoclasts. In disease states, the turnover can be increased (e.g., hyperparathyroidism) or decreased (e.g., hypoparathyroidism) with corresponding changes in blood and urinary calcium. The primary calcium regulating hormones that control this homeostatic system are PTH and vitamin D, which act at bone, kidney, and GI tract to increase serum calcium and to a lesser extent calcitonin, which decreases bone resorption, but does not appear to have a major effect on serum calcium under normal circumstances (10) (Figure 1).
What does the shaded area of the bone represent?
The dark vertical line between bone and ECF represents bone surface and bone-lining cells. Shaded area represents labile skeletal calcium. The various calcium compartments are not to scale. See text for discussion. (see Acknowledgements)
Why is calcium and phosphate important?
Calcium and phosphate are critical to human physiology (e.g. neuromuscular function) and are also needed for skeletal mineralization. An understanding of calcium and phosphate metabolism is required for the clinician to evaluate disorders of the levels of calcium and phosphorus as well as metabolic skeletal disorders.
Why is calcium important for bone health?
It is also a key electrolyte for blood clotting and the muscle contraction of both skeletal muscles and cardiac muscles. This is why consuming foods and drinks with high levels of calcium is often recommended.
What hormones are involved in calcium?
Parathyroid Hormone. Calcium is regulated by the parathyroid, which releases parathyroid hormone ( PTH ), as well as the kidneys. When calcium levels are low, PTH is released to break down bones and allow the calcium stored in the bones to be available in the bloodstream. PTH also activates vitamin D, which encourages additional calcium ...
Does calcium have a relationship with phosphorus?
Calcium has an inverse relationship to phosphorus. This means that as levels of phosphorus in the blood rise, levels of calcium in the blood fall because phosphorus binds to calcium reducing the available free calcium in the blood. On the other hand, calcium has a similar relationship to vitamin D, which means that when vitamin D rises, calcium also rises. Of note, the levels we are referring to are the levels of free calcium in the blood and not calcium stored in the bones.
Does calcium have a similar relationship to vitamin D?
On the other hand, calcium has a similar relationship to vitamin D, which means that when vitamin D rises, calcium also rises. Of note, the levels we are referring to are the levels of free calcium in the blood and not calcium stored in the bones.
Does PTH help with calcium?
PTH also activates vitamin D, which encourages additional calcium to be absorbed from the digestive tract and encourages the kidneys to retain more calcium while excreting phosphorus.
What are the main regulators of phosphate homeostasis?
The currently known main regulators of phosphate homeostasis include parathyroid hormone (PTH), calcitriol, and a number of peptides collectively known as the "phosphatonins" of which fibroblast growth factor-23 (FGF-23) has been best defined.
Does hypophosphatemia cause osteomalacia?
In the rest of the skeleton, hypophosphatemia will result in osteomalacia due to an insufficient formation of hydroxyapatite. This review will address phosphate metabolism and its role in bone health.
