How do you find the ionic charge of an isotope?
If protons outnumber electrons, the isotope has more positive charges than negative charges. In other words, the number of protons exceeds the number of electron by the same number as the positive charge. If the number of electrons exceeds the number of protons, the ion charge will be negative.
How do you read an isotope symbol?
0:277:31Isotope Notation - YouTubeYouTubeStart of suggested clipEnd of suggested clipThe number up here is called the mass number which is the number of protons. Plus the number ofMoreThe number up here is called the mass number which is the number of protons. Plus the number of neutrons. Don't make the mistake. And think that this is the number of neutrons.
How do you find the ion symbol?
Conventions for Writing Ions When writing the symbol for an ion, the one- or two-letter element symbol is written first, followed by a superscript. The superscript has the number of charges on the ion followed by a + (for positive ions or cations) or - (for negative ions or anions).
How do you find isotopes and ions?
0:213:44Worked example: Identifying isotopes and ions | Chemistry - YouTubeYouTubeStart of suggested clipEnd of suggested clipSo the sulfurs that have different number of neutrons those would be different isotopes. So in thisMoreSo the sulfurs that have different number of neutrons those would be different isotopes. So in this case we have 16 protons. And we have 16 neutrons. So if you add the protons. Plus the neutrons.
What do the numbers in an isotope symbol mean?
Each isotope of an element is characterized by an atomic number (the number of protons), a mass number (the total number of protons and neutrons), and an atomic weight (mass of atom in atomic mass units).
What is an isotopic symbol?
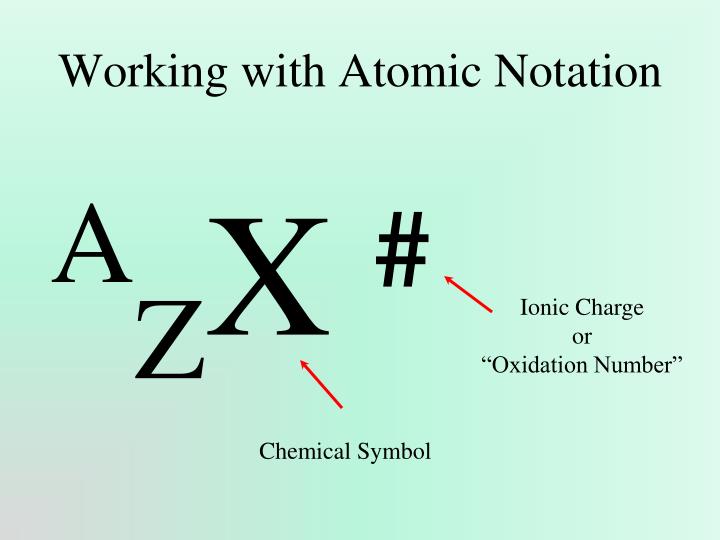
Isotopes can also be defined in standard, or "AZE", notation where A is the mass number, Z is the atomic number, and E is the element symbol. The mass number "A" is indicated with a superscript to the left of the chemical symbol "E" while the atomic number "Z" is indicated with a subscript.
How do you find a charge of an ion?
0:533:12How to Identify the Charge of an Ion : Chemistry Lessons - YouTubeYouTubeStart of suggested clipEnd of suggested clipSo if it's an ion it has eight already which means that it has one more electron than it has aMoreSo if it's an ion it has eight already which means that it has one more electron than it has a neutral charge. So a chloride a chlorine ion or chloride is going to have a charge of negative one.
What is the charge of ion?
ion, any atom or group of atoms that bears one or more positive or negative electrical charges. Positively charged ions are called cations; negatively charged ions, anions.
Which ion has a charge of 2+?
A magnesium atom must lose two electrons to have the same number electrons as an atom of the previous noble gas, neon. Thus, a magnesium atom will form a cation with two fewer electrons than protons and a charge of 2+. The symbol for the ion is Mg2+, and it is called a magnesium ion.
Can an isotope have a charge?
Can an isotope have a charge, i.e. the number of neutrons and electrons differ in that atom? If it does exists, is it correct to call it an ion isotope or isotope ion? Yes and yes. Isotope is just an atom; it does everything that normal atom does.
Is an isotope an ion?
An ion is an atom with a net electric charge due to the loss or gain of one or more electrons. An isotope is each of two or more forms of the same element that contain equal numbers of protons but different numbers of neutrons in their nuclei, and hence differ in relative atomic mass but not in chemical properties.
How do you tell if an isotope is an ion?
Isotopes involve neutrons. Answer: F-, Al3+, S2- , and SO42- are ions. If there is a negative or a positive sign after the element symbol(s) it is an ion. There is some overlap. For example, isotopes can lose or gain electrons to form ions.
What does it mean when the charge on an atom is zero?
When the charge on an atom is zero it means the number of protons is equal to the number of electrons. The atom is said to be neutral and there will not be a + or - written after the element symbol.
How are isotopes formed?
Formation of Isotopes and Ions. Isotopes can be made in supernovas, through radioactive decay of elements, and in specialized laboratories. The resulting isotopes have similar chemical and physical properties. In general it is difficult to make isotopes and involves a lot of energy. These are termed nuclear reactions.
Why are ion formations more common?
Ion formation is more common because electrons are on the outside of an atom and more easily added or removed.
What happens if there is a negative or positive sign after the element symbol?
There is some overlap. For example, isotopes can lose or gain electrons to form ions. Isotopes of Chlorine (Cl) gain an electron when they form ionic bonds. This results in a negative ion (the Chloride ion).
What are the two types of elements that have different numbers of neutrons?
Isotopes and Ions. Isotopes are versions of a particular element that have different numbers of neutrons. Ions are atoms (or molecules) that have lost or gained electrons and have an electrical charge. Isotopes involve neutrons.
What happens when an atom loses electrons?
When an atom loses electrons it becomes a positive ion (called a cation). For example, when Lithium-7 (Li) loses an electron it becomes Li +. Only the number of electrons changes. It is now the Lithium-7 cation. Exercise: Compete the table for the following atoms: Nuclear Notation. Mass Number. Atomic Number. Protons.
Does adding electrons change the mass number?
The effect on charge when adding electrons. Note: select "Atom" and then make sure you expand the "Net Charge" and "Mass Number" options. Adding electrons does not change the mass number.