Which of the following is the most stable cyclohexane form?
The chair conformation is more stable than the boat conformation. The boat conformation can sometimes be more stable than it is usually, by a slight rotation in the C-C bonds and is called the skew boat conformation. Nevertheless, the chair conformation is the most stable cyclohexane form.
Why is the boat conformation of cyclohexane not stable?
The boat conformation of cyclohexane is not a very stable form due to the torsional strain applied to the cyclohexane molecule. The stability of this form is further affected by steric interactions between the hydrogen atoms.
Which bond conformation is the least stable?
Boat conformation is the least stable, with the highest strength, has steric hindrance on carbon 1 and carbon 4 between the two equatorial hydrogens, and has torsional stress, as each bond almost fully ellipses other bonds in the Newman projection. Are diastereomers optically active?
Is cyclohexane planar or nonplanar?
In order to avoid the strain, cyclohexane does not exist as a planar molecule as expected. It exists as a puckered ring which is non-planar and the bond angles are close to tetrahedral bond angles. Two such puckered rings for cyclohexane called boat and chair conformations.
Which conformation is least stable for cyclohexane?
Half chair conformer is least stable due to maximum strain.
Which conformation is least stable?
Therefore, the correct option is option (D) twisted chair conformation.
Which conformation is most unstable in cyclohexane?
Half chair form is the most unstable conformation of cyclohexane.
Which among the conformations of cyclohexane is the most stable?
The chair conformationThe chair conformation is the most stable conformation of cyclohexane.
Why is half chair conformation least stable?
The half chair, formed by raising the footrest of the chair, has five of the six C atoms in a plane and one C atom out of the plane. Therefore, it has both eclipsing and bond angle strains and hence is the least stable conformation of cyclohexane.
Why is eclipsed conformation less stable?
hydrocarbons. …with respect to the other—the eclipsed conformation is the least stable, and the staggered conformation is the most stable. The eclipsed conformation is said to suffer torsional strain because of repulsive forces between electron pairs in the C―H bonds of adjacent carbons.
Why is axial conformer less stable?
Both chair conformations have one axial substituent and one equatorial substituent. According to the guideline, the conformer with the larger substituent in equatorial is more stable because if the large group is axial, a stronger steric strain will be generated and it is less stable.
Why is the boat conformation less stable?
The boat conformation suffers from torsional strain, making it less stable (higher in energy) than the chair. Steric strain in the boat arises mainly from the repulsion (steric crowding) between the two hydrogens on the ends of the "boat.
How do you know which conformation is most stable?
To find the most stable conformation, we choose the form with the least number of large axial groups; the least stable will have the most number of axial groups.
How do you know which conformation is most stable?
To find the most stable conformation, we choose the form with the least number of large axial groups; the least stable will have the most number of axial groups.
Is anti or gauche more stable?
The gauche form is less stable than the anti form due to steric hindrance between the two methyl groups but still is more stable than the eclipsed formations. Such an interaction is often referred to as a gauche-butane interaction because butane is the first alkane discovered to exhibit such an effect.
What is the least stable conformation of butane?
In butane the gauche-conformer is less stable than the anti-conformer by about 0.9 kcal/mol. This is due to a crowding of the two methyl groups in the gauche structure, and is called steric strain or steric hindrance.
Why is the boat conformation less stable?
The boat conformation suffers from torsional strain, making it less stable (higher in energy) than the chair. Steric strain in the boat arises mainly from the repulsion (steric crowding) between the two hydrogens on the ends of the "boat.
Which conformation of cyclohexane is chiral?
Cyclohexane conformation free of angle strain: chair conformation is achiral because it has a centre of symmetry because boat conformation is achir...
Which is the most stable conformation of cyclohexane?
The chair form shown to the right is the most stable conformation of cyclohexane. The C-C-C bonds are very similar to 109.5 o , so they are almost...
Which conformation of cyclohexane is the least stable?
Boat conformation is the least stable, with the highest strength, has steric hindrance on carbon 1 and carbon 4 between the two equatorial hydrogen...
Are diastereomers optically active?
Optical activity is the ability of a substance to rotate the plane polarised light. Chirality is the necessary condition for optical activity. Dias...
Which conformation is more stable, axial or equatorial?
Since axial bonds are parallel to each other, substituents larger than hydrogen typically suffer from greater steric crowding when axial rather tha...
What is the lowest energy conformation for cyclohexane?
This chair conformation is the lowest energy conformation for cyclohexane with an overall ring strain of 0 kJ/mol. In this conformation, the carbon-carbon ring bonds are able to assume bonding angles of ~111 o which is very near the optimal tetrahedral 109.5 o so angle strain has been eliminated.
Which cycloalkane has the least angle strain?
We will find that cyclohexanes tend to have the least angle strain and consequently are the most common cycloalkanes found in nature. A wide variety of compounds including, hormones, pharmaceuticals, and flavoring agents have substituted cyclohexane rings.
How is cyclohexane boat conformation created?
The Boat Conformation of cyclohexane is created when two carbon atoms on opposite sides of the six-membered ring are both lifted up out of the plane of the ring creating a shape which slightly resembles a boat. The boat conformation is less stable than the chair form for two major reasons. The boat conformation has unfavorable steric interactions between a pair of 1,4 hydrogens (the so-called "flagpole" hydrogens) that are forced to be very close together (1.83Å). This steric hindrance creates a repulsion energy of about 12 kJ/mol. An additional cause of the higher energy of the boat conformation is that adjacent hydrogen atoms on the 'bottom of the boat' are forced into eclipsed positions. For these reasons, the boat conformation about 30 kJ/mol less stable than the chair conformation.
What is the ring flip of cyclohexane?
Cyclohexane is rapidly rotating between the two most stable conformations known as the chair conformations in what is called the "ring flip" shown below. The importance of the ring flip will be discussed in the next section.
What is the color of the flagpole hydrogens in cyclohexane?
A boat structure of cyclohexane (the interfering "flagpole" hydrogens are shown in red)
Is C-H ring staggered?
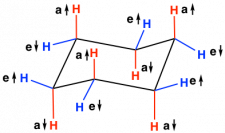
Also, the C-H ring bonds are staggered so torsional strain has also been eliminated. This is clearly seen when looking at a Newman projection of chair cyclohexane sighted down the two central C-C bonds.
Is cyclohexane a planar structure?
A planar structure for cyclohexane is clearly improbable. The bond angles would necessarily be 120º, 10.5º larger than the ideal tetrahedral angle. Also, every carbon-hydrogen bond in such a structure would be eclipsed. The resulting angle and eclipsing strains would severely destabilize this structure. The ring strain of planar cyclohexane is in excess of 84 kJ/mol so it rarely discussed other than in theory.
How many substituents does cyclohexane have?
Cyclohexane can have more than two substituents. Also, there are multiple six membered rings which contain atoms other than carbon. All of these systems usually form chair conformations and follow the same steric constraints discussed in this section. Because the most commonly found rings in nature are six membered, conformational analysis can often help in understanding the usual shapes of some biologically important molecules. In complex six membered ring structures a direct calculation of 1,3-diaxial energy values may be difficult. In these cases a determination of the more stable chair conformer can be made by empirically applying the principles of steric interactions.
How many chair conformations does a cyclohexanes have?
Based on the table above, cis -1,4-disubstitued cyclohexanes should have two chair conformations each with one substituent axial and one equatorial. Based on this, we can surmise that the energy difference of the two chair conformations will be based on the difference in the 1,3-diaxial interactions created by the methyl and chloro substituents.
How to determine stable chair conformation?
To determine the stable chair conformation, the steric effects of each substituent, along with any additional steric interactions, must be taken into account for both chair conformations.
Why do substitutes prefer equatorial positions?
Substituents prefer equatorial rather than axial positions in order to minimize the steric strain created of 1,3-diaxial interactions.
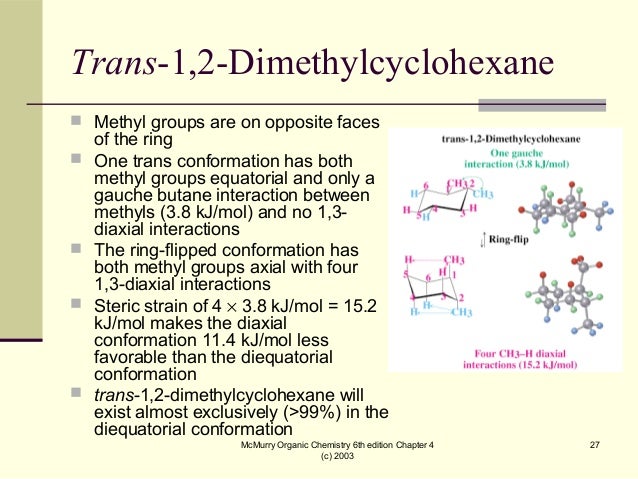
How many kJ/mol of steric strain is created by a gauche interaction between the two methyl?
It is important to note, that both chair conformations also have an additional 3.8 kJ/mol of steric strain created by a gauche interaction between the two methyl groups. Overall, both chair conformations have 11.4 kJ/mol of steric strain and are of equal stability.
How to determine if a chair is more stable?
The more stable chair conformation can often be determined empirically or by using the energy values of steric interactions previously discussed in this chapter. Note, in some cases there is no discernable energy difference between the two chair conformations which means they are equally stable.
Which conformation is more stable?
The other conformer places both substituents in equatorial positions creating no 1,3-diaxial interactions. This diequatorial conformer is the more stable regardless of the substituents.