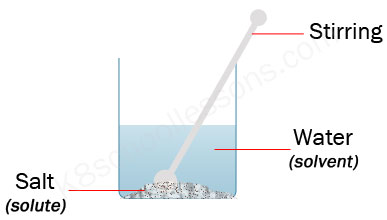
Does salt dissolve quicker than sugar?
Sugar dissolves faster,But salt can dissolve faster in hot water than sugar.Sugar dissolves faster than salt in certain types of liquid like cold water thanked the writer.
Does salt melt faster than sugar?
Salt will melt ice faster than the same amount of sugar because there are more molecules in salt than there are in sugar. It is the colligative property of salt that makes ice melt faster. Pure water has a freezing point that is constant. It is 32 degrees Fahrenheit.
Does sugar have a higher solubility than salt?
You can see that at all temperatures, many more grams of sugar dissolve than salt. The graph also shows that the solubility of sugar increases much more than the solubility of salt as the temperature of the water increases. Alum is the least soluble until the temperature of the water increases to about 65 °C.
What is the fastest way to dissolve sugar?
- Increase the surface area of the sugar.
- Increase the temperature of the water.
- Stir it up.

Why does sugar dissolve faster than salt?
Therefore, sugar dissolves faster than salt, because its component molecules are more soluble in water.
Is salt a compound or a compound?
Salt is a compound of sodium and chloride, and the molecular ionic bond that joins these particles together is very strong. Table sugar is composed of glucose and fructose molecules, but the positive-negative bond is weaker between these components. When dissolved, sugar molecules have more opportunities to bond with positive-negative water ...
Does sugar dissolve faster than salt?
Nope Jemmax. Sugar dissolves faster than salt in water. Salt has stronger bonds than sugar. That what makes sugar dissolve faster (because it has weaker bonds and structure than salt).
Can sugar dissolve in water?
If sugar and salt are dissolving in the polar substance like water then salt can dissolve earlier because it is ionic and polar in nature so it +ve and-ve ions dispersed in water.but sugar is non-polar and water will only dissolve only -oH part of sugar.and if they are dissolving in non-polar substance like oil then vice versa.
Why does salt dissolve faster?
But normally salt dissolve faster because it's constituents sodium and chlorine dissociate faster as compared to the sugar Crystal made of carbon hydrogen and oxygen.
Which gets dissolved the fastest?
while u can know the answer by physically trying to dissolve both and u will find it's salt which gets dissolved the fastest..
Why are salts soluble in water?
Salts are soluble in water due to high dielectric constant of water by the virtue of which charge separation becomes easier. Sugar is a non polar covalent compound wheras water is polar. Hydrogen and oxygen atoms present in sugar can form hydrogen bonds with water molecules. 2.8K views.
What ions does water dissociate from NaCl?
Water dissociated the NaCl into Na+ and Cl- ions.
What units will you use for solubility?
What units will you use: grammes or moles? Traditionally, solubility is expressed as g/ 100ml, while rate processes are in terms of molarity.
Why is water polar?
Essentially, if the dipole moments of the solute and solvent are similar, the solute dissolve. Water is very polar. There is said to be a partial negative charge at the oxygen end and a partial positive charge at the hydrogen. This is due to oxygen’s electronegativity being higher than hydrogens.
What happens when you dump a solute into a solvent?
If you dump a solute into a solvent, you get a concentration gradient - high near the solid and practically zero far away and you have to rely on natural diffusion to achieve a uniform solution. This can be seen with any coloured solute, even with a liquid dye. So, you need to stir the mixture and the stirring rate is a factor.