Its underlying mechanism was discovered 82 years ago by the British chemists Edward Hughes and Christopher Ingold, who showed that an electron-rich chemical species, called a nucleophile, "attacks" and replaces an electron-poor fragment of an organic molecule, called a leaving group.
What is the difference between SN1 and SN2?
SN1 reactions are nucleophilic substitutions, involving a nucleophile replacing a leaving group (just like SN2). However: SN1 reactions are unimolecular:the rate of this reaction depends only on the concentration of one reactant. ●SN1 reactions happen in two steps:
What is SN1 reaction in organic chemistry?
1 Reaction. SN1 reactions are nucleophilic substitutions, involving a nucleophile replacing a leaving group (just like SN2). However: SN1 reactions are unimolecular: the rate of this reaction depends only on the concentration of one reactant.
What is SN2 reaction give an example?
SN 2 reaction is also known as bimolecular nucleophilic substitution reaction. Such reactions are generally shown by primary haloalkanes. For example, hydrolysis of ethyl bromide with aq.KOH. Rate of SN 2 reaction depends upon the concentration of both substrate (i.e. alkyl halide) and nucleophile.
Why do we call it SN1?
Another way of saying this is that the reaction is “unimolecular,” and this is why we call it Sn1: Substitution – nucleophilic – unimolecular.

Who discovered SN1 and SN2 mechanism?
Hughes and Ingold initiated reactionkinetic studies on nucleophilic substitution in the 1930s which matured to a grand-scale understanding of SN1 and SN2 reaction mechanisms in 1953 when Ingold published his classic book on “Structure and Mechanism in Organic Chemistry”.
Who discovered SN2 reaction?
Hughes and Ingold first made the observation, subsequently confirmed by generations of chemists, that SN2 reactions all seemed to occur via “backside attack”, whereby the nucleophile joins the organic molecule at a location opposite to the leaving group.
Who discovered substitution reaction?
11.1 MECHANISMS OF SUBSTITUTION REACTIONS In 1933, Christopher (later Sir Christopher) Ingold with Patel [1,2] introduced a classification for substitution reactions on carbon atoms that was subsequently popularised by Ingold and E. D. Hughes.
Who invented nucleophilic substitution?
Further, in 1930, Sir Christopher Ingold described for the first time two different forms of nucleophilic substitution reactions, which are known as SN1 (nucleophilic substitution unimolecular) and SN2 (nucleophilic substitution bimolecular) reaction.
Who discovered SN1 reaction?
Christopher Ingold etIn inorganic chemistry, the SN1 reaction is often known as the dissociative substitution. This dissociation pathway is well-described by the cis effect. A reaction mechanism was first proposed by Christopher Ingold et al. in 1940.
Why is it called SN1?
SN 1 reactions' rates are only dependent on on entity, the electrophile (loss of a leaving group is the first step of this reaction and it does not require a nucleophile at first to have the Leaving Group leave and form carbocation), therefore it is called SN1.
Who discovered mechanism?
In organic chemistry, the reaction mechanism for the benzoin condensation, put forward in 1903 by A. J. Lapworth, was one of the first proposed reaction mechanisms.
What is SN1 and SN2 reactions in organic chemistry?
A nucleophilic substitution reaction is a reaction that involves the replacement of one functional group or atom with another negatively charged functional group or atom. SN1 is a unimolecular reaction while SN2 is a bimolecular reaction. SN1 involves two steps. SN2 involves one step.
What is the difference between SN1 and SN2 reaction?
To understand the difference between SN1 and SN2, it is important to know their definitions first....Difference between SN1 and SN2The rate of reaction is unimolecular.The rate of reaction is bimolecularIt is a two-step mechanismIt is only a one-step mechanism4 more rows
Why SN2 is called bimolecular?
Biomolecular Nucleophilic Substitution Reactions and Kinetics. In the term S N2, the S stands for substitution, the N stands for nucleophilic, and the number two stands for bimolecular, meaning there are two molecules involved in the rate determining step.
What is the rate law for SN1 reaction?
rate = k [substrate]. According to the rate law, an SN1 reaction is first order overall, and the concentration of the nucleophile does not affect the rate.
What is SN1 mechanism in organic chemistry?
SN1 reaction mechanism follows a step-by-step process wherein first, the carbocation is formed from the removal of the leaving group. Then the carbocation is attacked by the nucleophile. Finally, the deprotonation of the protonated nucleophile takes place to give the required product.
What is an SN2 reaction in organic chemistry?
The SN2 reaction is a type of reaction mechanism that is common in organic chemistry. In this mechanism, one bond is broken and one bond is formed synchronously, i.e., in one step. SN2 is a kind of nucleophilic substitution reaction mechanism, the name referring to the Hughes-Ingold symbol of the mechanism.
What does SN2 stand for?
Substitution Nucleophilic BimolecularThe term 'SN2' stands for – Substitution Nucleophilic Bimolecular. This type of reaction is also referred to as bimolecular nucleophilic substitution, associative substitution, and interchange mechanism.
How are SN2 reactions used in the real world?
The SN2 reaction can be used to detach part of a molecule called a functional group from a central carbon atom, while simultaneously, another functional group adds to the opposite side of the carbon atom. This structural flip can significantly change a compound's chemical properties.
How do SN2 reactions occur?
In the SN2 reaction, the nucleophile approaches the carbon atom to which the leaving group is attached. As the nucleophile forms a bond with this carbon atom, the bond between the carbon atom and the leaving group breaks. The bond making and bond breaking actions occur simultaneously.
Nucleophilic Substitution Unimolecular S N 1
S N 1 reactions are proceeded by the ionization mechanism. It is a two-step process. Ionization occurs in the first step that is very slow. The rate-determining heterolytic dissociation of the reactant results in a carbocation and leaving group. This dissociation occurs due to the combination of the electrophilic carbocation with a nucleophile.
Nucleophilic Substitution Bimolecular S N 2
S N 2 reactions are followed by the direct displacement mechanism. It completes the reaction in a single step that is rate-determining (Transition State). According to this mechanism, the reactant is attacked by the nucleophile (Lewis base) from the opposite side of the leaving group.
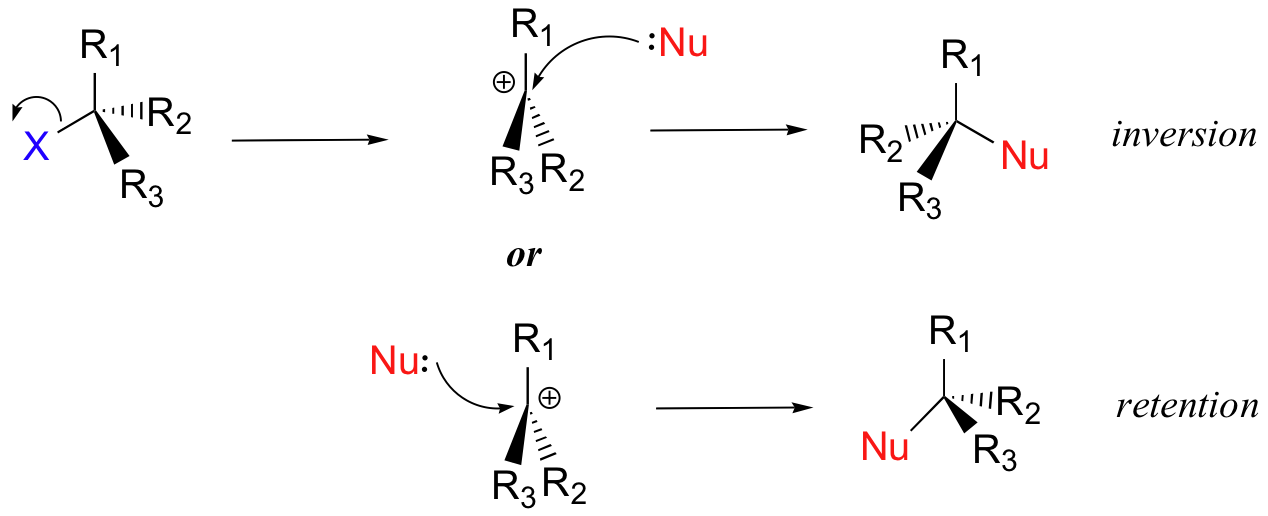
What is the stereochemistry of SN1?
Stereochemistry of SN1 reaction: In SN 1 reaction, carbocations are formed as the intermediate which are trigonal and planar. Carbocation has a flat structure so that nucleophile can attack it from either side (i.e. front or back) resulting in the formation of two products, one with retention of configuration and other with inversion ...
What is SN in chemistry?
Nucleophilic substitution (SN) reaction. Nucleophiles are electron rich atoms or group of atoms which attack on electron deficient centre during chemical reaction. Nucleophiles are the negatively charged species or neutral species having electron rich centre. Any substitution reaction that involves replacing of an atom or a functional group by ...
What is the name of the reaction in which a nucleophile attacks a halogen atom?
During nucleophilic substitution reaction in haloalkanes (alkyl halides) , the nucleophile attacks the haloalkane and replaces the halogen atom.
What is the first step of the carbon-halogen bond?
Step I : In first step, the carbon-halogen bond of tertiary butyl bromide slowly breaks heterolytically to form an intermediate carbocation i.e. tert-butyl carbocation.
Which atom is attacked by the nucleophile?
It is assumed that the nucleophile attacks the carbon atom attached to the halogen atom from the side opposite to the halogen (i.e. backside attack). As a result a transition state (activated complex) is formed in which carbon atom is partially bonded to both nucleophile and leaving group (halogen atom).
What are the two types of nucleophilic substitution reactions?
There are two main types of nucleophilic substitution reactions – SN 2 and SN 1 reaction.
Why do Sn1 and Sn2 react?
Sn1: Sn1 reactions tend to happen in polar, protic solvents, because they can stabilize the carbocation charge better through their strong solvating power. This essentially means that the protic solvent can surround the charge and interact with it, which stabilizes the charge.
How to determine if an electrophile is a sn1 or sn2 reaction?
Thus, the structure of the electrophile is the easiest way to determine in a reaction will proceed via sn1 vs. sn2. If the leaving group is attached to a primary or tertiary carbon, in most cases you can automatically assume an sn1 or sn2 reaction, respectively. If it is attached to a secondary carbon, the case is a little more ambiguous. You may have to rely on other clues to determine which reaction it will be. In these cases, look at the nucleophile (whether it is charge/uncharged, or strong/weak), and at the solvent (whether it is protic or aprotic).
What is the most significant difference between Sn1 and Sn2?
The position of the leaving group on the electrophile is perhaps the most significant when it comes to distinguishing between sn1 vs. sn2 reactions.
What is the ion that detaches from the molecule during the reaction?
Carbocation: an ion with a positively charged carbon. Leaving group: the atom or group of atoms that detach from the molecule during the course of the reaction. Protic (solvent): a solvent that contains a hydrogen bonded to an oxygen, nitrogen, or fluorine atom, which can serve as a source of H + atoms.
What is the slowest step in a reaction?
The slowest step in a reaction is the one that limits the rate of the overall reaction, just like the neck of a bottle determines how quickly you can pour out its contents. In an Sn1 reaction, this slowest step is the dissociation of the electrophile, when the leaving group leaves.
What is the rate equation for Sn2?
Similarly, because TWO reactants must come together in the rate determining (and only) step of an Sn2 reaction, we call this type of reaction “bimolecular” and write its rate equation as R = k [electrophile] [nucleophile]. This leads to the name Sn2: Substitution – nucleophilic – bimolecular.
Do polar solvents hinder SN2 reactions?
This makes sense, as they do not have to stabilize a carbocation in sn2 reactions. In fact, too strong a solvating power, such as the polar, protic solvents, will hinder sn2 reactions because it will solvate the nucleophile, and prevent it from “attacking” the electrophile.
What is the steric hindrance of S N 2?
This phenomenon, called "steric hindrance," imposes strict limits on how rapidly S N 2 reactions can happen. By contrast, the newly discovered reaction, which the researchers call S N 2X, occurs via frontside attack and is not prone to steric hindrance.
What is the name of the nucleophilic substitution reaction?
One of the main types of nucleophilic substitution reactions, called S N 2 , involves the nucleophile attacking and the leaving group departing at the same time. Hughes and Ingold first made the observation, subsequently confirmed by generations of chemists, that S N 2 reactions all seemed to occur via "backside attack," whereby the nucleophile joins the organic molecule at a location opposite to the leaving group.
What does the absence of steric hindrance in S N 2X mean?
The absence of steric hindrance in S N 2X means that certain reactions in organic chemistry can be performed more efficiently than previously believed. "In the paper, we demonstrated the S N 2X reaction in a specially chosen set of reactions – enantioselective reactions of sterically hindered tertiary halides," explained Professor Tan. "But now that one example has been found, it seems very likely that others will follow. The revision of such a foundational part of organic chemistry means that many reactions, which chemists thought we understood, might now have to be re-examined. This could have wide-ranging implications throughout the field."
What is nucleophilic substitution?
Nucleophilic substitution is a class of chemical reactions encountered throughout organic chemistry, including those used to manufacture common petrochemical and pharmaceutical products. Its underlying mechanism was discovered 82 years ago by the British chemists Edward Hughes and Christopher Ingold, who showed that an electron-rich chemical species, called a nucleophile, "attacks" and replaces an electron-poor fragment of an organic molecule, called a leaving group.
Comparison of SN1 vs SN2 Reactions
The product is a racemic mixture i.e. 50% inversion and 50% retention of configuration.
Some Important Points About SN1 Reaction
SN1 reaction depends upon the nature of alkyl halide. The nature of alkyl halide means the formation of the most stable carbocation. The order of stability of carbocation is:
SN1 Reaction
Means of SN1 reaction is uni molecular nucleophilic substitution reaction. It means that molecularity of reaction is 1. As we know that the molecularity of reaction depending up on the number of reactants molecule which are participating in rate determining step. or slow step.
Reaction Mechanism of SN1 Reaction
First step is rate determine step in which the formation of carbo-cation take place after removal of leaving group halogen atom.
Stereo Chemistry of SN1 Reaction
The product which will form after SN1 reaction having either retention or inversion in configuration.
SN2 Reaction
Means of SN2 reaction is biomolecular nucleophilic substitution reaction. It means that molecularity reaction is 2. As you know that the molecularity of reaction depending up on the number of reactant molecules which are participating in rate determining step or slow step.
Reaction Mechanism of SN2 Reaction
Product formation take place by a transition state in which old bond breaking and new bond forming both take place simultaneously.
Stereo Chemistry of SN2 Reaction
In the stereo chemistry of SN2 reaction. it has been concluded that's newly produce product formed always has inversion in configuration in the owner of the scientist walden sach inversion is known as walden inversion.

CORE Concepts
Related Topics
- SN2 reactions are followed by the direct displacement mechanism. It completes the reaction in a single step that is rate-determining (Transition State). According to this mechanism, the reactant is attacked by the nucleophile (Lewis base) from the opposite side of the leaving group. During the reaction, bonds are broken down and new bonds are forme...
Vocabulary
SN1 vs. SN2 Rate Equations
- In this tutorial, you will learn how to explicitly distinguish between the different aspects of sn1 vs. sn2 reactions, and to identify the factors that make each more likely to occur.
SN1 vs. SN2 Electrophiles
SN1 vs. SN2 Nucleophiles
- Aprotic (solvent): a solvent that does not contain hydrogen atoms bonded to oxygen, nitrogen, or fluorine, and thus cannot hydrogen bond. It may contain hydrogen atoms elsewhere, such as bonded to...
- Carbocation:an ion with a positively charged carbon.
- Leaving group: the atom or group of atoms that detach from the molecule during the course o…
- Aprotic (solvent): a solvent that does not contain hydrogen atoms bonded to oxygen, nitrogen, or fluorine, and thus cannot hydrogen bond. It may contain hydrogen atoms elsewhere, such as bonded to...
- Carbocation:an ion with a positively charged carbon.
- Leaving group: the atom or group of atoms that detach from the molecule during the course of the reaction.
- Protic (solvent): a solvent that contains a hydrogen bonded to an oxygen, nitrogen, or fluorine atom, which can serve as a source of H+atoms. This is a solvent that has the ability to hydrogen bond.
SN1 vs. SN2 Solvents
- The numbers associated with Sn1 and Sn2 reactions can seem counterintuitive at first. If you think about the number of steps involved in these reactions, they seem backwards. However, the numbers refer to the number of reactants involved in the rate-determining step, not to the number of steps. The slowest step in a reaction is the one that limits the rate of the overall reaction, just l…
SN1 vs. SN2 Leaving Groups
- The position of the leaving group on the electrophile is perhaps the most significant when it comes to distinguishing between sn1 vs. sn2 reactions. Sn1: if the leaving group is attached to a tertiary carbon, it is most likely to undergo an sn1 reaction; if attached to a secondary carbon, less likely, and if attached to a primary carbon, very unlikely – essentially impossible. This is because the fir…
Summary
- Sn1: In sn1 reactions, the nucleophile tends to be uncharged and weaker, as it is “attacking” a carbocation. This means that it will not take very much strength for the second step, the nucleophilic attack, to occur – the charge of the electrophile encourages it already. Often, in an sn1 reaction, the nucleophile isthe solvent that the reaction is occurring in. Some examples of nu…