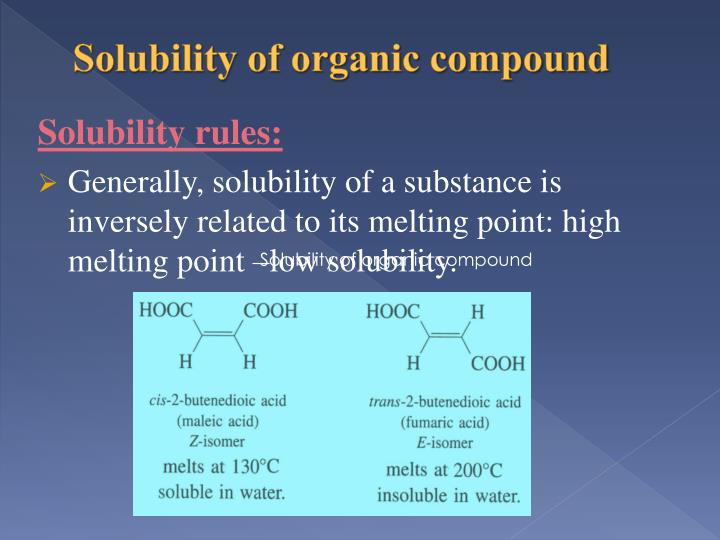
What happens to the solubility of alcohol in water as the hydrocarbyl tail grows?
Is methanol a solvent?
About this website
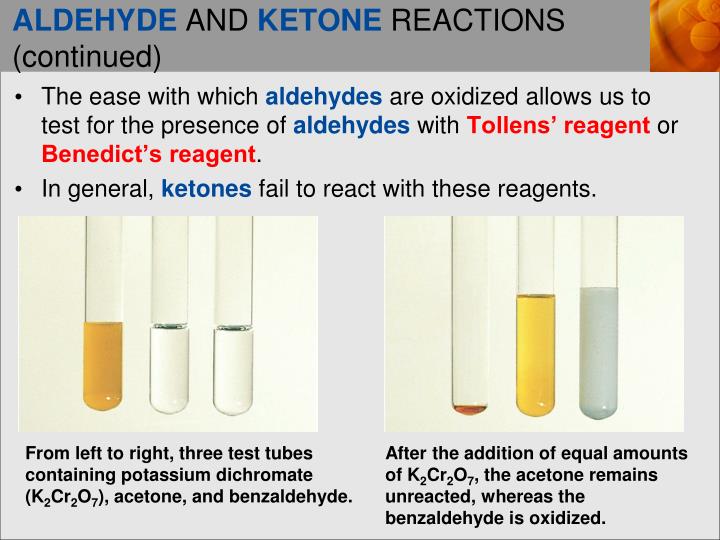
Why are low molecular alcohols are soluble in water?
MSo we can say that lower alcohols are soluble in water due to the formation of hydrogen bonding with the water molecule.
Are small molecular weight alcohols soluble in water?
Alcohols of low molecular weight are soluble in water while that of high molecular weight are insoluble in water . because of high hydrogen bonding in low molecular weight Alcinous.
Why the low molecular weight alcohols are more soluble in water than the low molecular weight ethers?
Therefore, due to the high electronegativity of oxygen, it can form hydrogen-bond with water molecules similar to alcohol. But due to the absence of H on O of ether, they can only act as hydrogen bond acceptor (unlike alcohols) and thus have lower solubility in water than alcohols with similar molecular weight.
Why are lower molecular weight alcohols more soluble in water than higher molecular weight alcohols?
With the increase in the molar mass, solubility decreases. In alcohols, −OH group is polar and forms hydrogen bonds with water. This makes alcohol water soluble.
How does molecular weight affect solubility in water?
Within alkanes, molecular size is the primary determinant of their solubility in water, and increasing molecular size results in a decrease in water solubility mainly due to the increased free energy penalty for cavity formation in water.
Why is alcohol easily soluble in water?
As alcohol is a polar solvent . It form Hydrogen Bond with water molecules while other hydrocarbons of comparable molecular masses does not form Hydrogen Bond due to being non - polar . Hence Alcohol is more soluble in water.
Why low weight alcohols are soluble in water but higher weight alcohols are not?
Alcohols with higher molecular weights tend to be less water-soluble, because the hydrocarbon part of the molecule, which is hydrophobic (“water-hating”), is larger with increased molecular weight.
Why the low molecular weight ethers are water soluble?
Solubility. Ethers containing up to 3 carbon atoms are soluble in water, due to their hydrogen bond formation with water molecules. The solubility decreases with increase in the number of carbon atoms. The relative increase in the hydrocarbon portion of the molecule decreases the tendency of H-bond formation.
Is lower molecular mass alcohols are miscible in water in all proportions?
Explanation : Lower molecular mass alcohols are able to form hydrogen bonds with water. Thus are miscible in all proportions.
Why are low molecular weight carboxylic acids soluble in water?
The carboxyl group readily engages in hydrogen bonding with water molecules (Figure 15.3. 1). The acids with one to four carbon atoms are completely miscible with water.
Why are smaller alcohols more polar?
Explanation: Small alcohols have attached OH groups which make the alcohols polar.
Does solubility of alcohol increase with molecular weight?
The solubility of alcohols in water gradually increases with molecular weight.
How does molecular weight affect dissolution?
As the molecular weight of the solute increases, the value of Ω decreases, and, as a result the entropic driving forces are diminished for dissolution of polymers. As a consequence, polymer solubility depends strongly on the enthalpic interaction between the polymer and the solvent.
Which alcohol is more soluble in water Why?
With the increase in the molar mass, solubility decreases. In alcohols, −OH group is polar and forms hydrogen bonds with water. This makes alcohol water soluble.
Does solubility depend on molecular weight?
It is well-known from the literature that the solubility properties of the polymers depend on their molecular weight, therefore, the solubility of the polyester samples with increasing molecular mass was also studied by precipitation method as shown in Figure 7.
Are high molecular weight alcohols water soluble?
But alcohols with higher molecular weights are less miscible in water because as the weight increases the hydrocarbon part which is hydrophobic(water-hating) becomes larger making it less miscible.
Why are smaller molecules more soluble?
Additionally, solutes will be more soluble if the molecules in the solute are smaller than the ones in the solvent. This is because it is more difficult for solvent molecules to surround bigger molecules.
Why is ethanol most soluble in water?
Ethanol has an overall polarity that allows it to make hydrogen bonds to water easily, allowing solubility. Methanol, ethanol and propanol have just less enough hydrocarbon chains to make the -OH to H2O bond possible (i.e. soluble).
Why ethanol is easily dissolve in water?
Ethanol has a hydroxyl(-OH) functional group in it. Due to the presence of a bond between electronegative atom oxygen and hydrogen, the O-H bond acquires polarity. Hence, due to the presence of polarity in O-H bond, it forms a Hydrogen bond with water which is responsible for the responsibility of ethanol in water.
Are alcohols the most soluble in water?
Because of the strength of the attraction of the OH group, first three alcohols (methanol, ethanol and propanol) are completely miscible. They dissolve in water in any amount....Alcohol solubility chart.NameFormulaSolubilityMethanolCH3OHmiscibleEthanolC2H5OHmisciblePropanolC3H7OHmiscibleButanolC4H9OH0.113 more rows
Are smaller molecules more soluble in water?
Molecular size If all of the above mentioned factors ale excluded, a general rule can be found that larger particles are generally less soluble. If the pressure, and temperature are the same than out of two solutes of the same polarity, the one with smaller particles is usually more soluble.
Does solubility of alcohol increase with molecular weight?
The solubility of alcohols in water gradually increases with molecular weight.
Are high molecular weight alcohols water soluble?
But alcohols with higher molecular weights are less miscible in water because as the weight increases the hydrocarbon part which is hydrophobic(water-hating) becomes larger making it less miscible.
Is the short chain alcohols are soluble in water?
Small alcohols are completely soluble in water; mixing the two in any proportion generates a single solution. However, solubility decreases as the length of the hydrocarbon chain in the alcohol increases.
Why is ethanol more soluble in water than methanol? - Quora
Answer (1 of 10): Methanol. ethanol, and propanol are all completely miscible with water. That means that any of these alcohols can be mixed with water, in any proportion, and a true solution will be formed (the mixture will not separate into a water layer and an alcohol layer). If there is more ...
What is the meaning of an insoluble solid? - Quora
Answer (1 of 4): There is actually no such thing as an insoluble or soluble solid. The usual reference to a solvent is water and so when someone says a solid is insoluble, it means that it will not dissolve in water. However just because the given solid is insoluble in water doesn't mean it will ...
hyvonen.bioc.cam.ac.uk
hyvonen.bioc.cam.ac.uk
National Center for Biotechnology Information
National Center for Biotechnology Information
Dialysis strategies for protein refolding: preparative streptavidin ...
We have investigated different dialysis strategies for the refolding of recombinant streptavidin, and present a novel dialysis setup featuring gradual dilution dialysis and continuous protein feeding into a dialysis sack. A denaturing dialysis buffer is exchanged gradually by dilution with refolding …
Why is alcohol soluble in water?
Alcohols are soluble in water. This is due to the hydroxyl group in the alcohol which is able to form hydrogen bonds with water molecules. Alcohols with smaller hydrocarbon chain are very soluble. As the length of the hydrocarbon chain increases, the solubility in water decreases.
Why are alcohols more soluble in nonpolar solvents?
The longer the hydrocarbon chain, a higher percentage of the molecule becomes nonpolar and contributes to its insolubility. This is the exact same reason why longer chain alcohols are more soluble in nonpolar solvents.
How does the length of a hydrocarbon chain affect the solubility of water?
As the length of the hydrocarbon chain increases, the solubility in water decreases. With four carbon in the hydrocarbon chain and higher, the decrease in solubility becomes visible as the mixture forms two immiscible layers of liquid.
Why does solubility decrease as the length of the hydrocarbon chain increases?
The reason why the solubility decreases as the length of hydrocarbon chain increases (non-polar of alkane dominates) is because it requires more energy to overcome the hydrogen bonds between the alcohol molecules as the molecules are more tightly packed together as the size and matter increases. Deepak Kadian.
How many carbons are in ethanol?
Ethanol has a two carbon chain with one hydroxyl while decanol has ten carbons with one hydroxyl. This hydrocarbon chain is nonpolar, and has a high free energy change associate s with its dissolution in water. Simply put, dissolving a nonpolar substance in a polar solvent requires energy.
Why is the hydrocarbon chain smaller?
With the lower alcohols, the smaller length of the hydrocarbon chain allows the bonding between the alcoholic -OH and the water molecules to happen effectively . However, with an increase in the length of the hydrocarbon chain, the steric factor comes into picture thereby hindering the aforementioned interaction and consequently their solubility in water.
What happens when you mix alcohol and water?
There are two things going on (at least) when you mix an alcohol with water. The hydroxyl part of the alcohol forms hydrogen bonds very readily with water molecules. However, the hydrocarbon part (the rest of the chain) is almost completely insoluble in water — there are almost no favorable interactions, so dragging a hydrocarbon into water requires a lot of energy.
What happens to the solubility of alcohol in water as the hydrocarbyl tail grows?
As the hydrocarbyl tail GROWS, the solubility of the alcohol in water decreases. Propanol is the cut-off point; butanol, and the higher alcohols exhibit limited water solubility. For longer chain alcohols, non-polar interaction between the hydrocarbyl chains becomes more significant as an intermolecular force.
Is methanol a solvent?
Explanation: Both, say methanol, and ethanol, are water-like solvents...they are in effect HALF a water molecule..and reasonably, both alcohols are infinitely miscible with water. Methanol, in particular, is INSOLUBLE in hexanes. The influence of the hydroxyl group confers this water solubility and hexanes insolubility.
