Is water polar or nonpolar?
Here's how it works for water. Water (H 2O) is polar because of the bent shape of the molecule. The shape means most of the negative charge from the oxygen on side of the molecule and the positive charge of the hydrogen atoms is on the other side of the molecule.
Why is water (H2O) polar?
Because it has a net dipole moment. Explanation: Water #(H_2O)# is a polar molecule because the electrons of the hydrogen atoms get "pulled" towards the electrons of the oxygen atom. This makes a region of positive charge on the hydrogen atoms and the negative charge on the other end of the molecule, which is the oxygen atom. Have a look here:
Why is water a polar solvent?
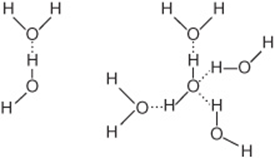
Why Water Is a Polar Solvent. The slight negative charge near the oxygen atom attracts nearby hydrogen atoms from water or positive-charged regions of other molecules. The slightly positive hydrogen side of each water molecule attracts other oxygen atoms and negatively-charged regions of other molecules.
What determines the polarity of water?
It's the movement of electrons that determines polarity. Here's how it works for water. Water (H 2O) is polar because of the bent shape of the molecule. The shape means most of the negative charge from the oxygen on side of the molecule and the positive charge of the hydrogen atoms is on the other side of the molecule.

Why are water molecules dipoles?
Water is a dipolar molecule because each atom has a dipole, or partial charge. Oxygen is more electronegative than hydrogen and thus pulls the shared electrons in the covalent bond closer toward its nucleus. This gives oxygen a partial negative charge and hydrogen a partial positive charge.
What is the meaning of water is dipolar?
Water is dipolar in nature. Hence when water enters an electric field, the molecules rearrange themselves. The case 1 in the figure, where a positively charged rod is brought near the water, the negative charge moves towards the rod and positive charge moves away from the rod.
Why is water considered a dipolar molecule quizlet?
Why is water a dipolar molecule? The oxygen is slightly negative and the hydrogens are slightly positive.
What makes a molecule dipolar?
Dipolar or polar molecules are the molecules that posses an electric dipole. The dipoles of some molecules depend on their environment and can change substantially when they are transferred from one medium to another, especially when molecules become ionized in a solvent.
Why does the water bend toward the rod?
When a negatively-charged rod is brought close to a water stream, individual water molecules align themselves so the positive (hydrogen) end points towards the rod. Negative and positive charges attract one another and the water stream is bent towards the rod.
What are the properties of water?
Unique properties of waterWater is polar. ... Water is an excellent solvent. ... Water has high heat capacity. ... Water has high heat of vaporization. ... Water has cohesive and adhesive properties. ... Water is less dense as a solid than as a liquid.
Why is water considered a polar molecule Brainly?
Answer. Water (H2O) is polar because of the bent shape of the molecule. The most of the negative charge from the oxygen on side of the molecule and the positive charge of the hydrogen atoms is on the other side of the molecule.
Why is water considered a water molecule?
A water molecule consists of two atoms of hydrogen linked by covalent bonds to the same atom of oxygen. Atoms of oxygen are electronegative and attract the shared electrons in their covalent bonds.
Why is water considered polar Quizizz?
The unequal sharing of electrons gives the water molecule a slight negative charge near its oxygen atom and a slight positive charge near its hydrogen atoms. The molecule has two poles, at which the it is colder than other regions of the molecule.
How do you know if a molecule is dipolar?
Dipoles can be determined by comparing the electronegativity of the bonded atoms. Arrows are used to indicate dipoles; arrows point towards the more electronegative atom. A dipole moment occurs when there is an overall uneven distribution of electrons across a molecule.
Is water polar or dipolar?
2: Water is a polar molecule, as greater electron density is found around the more electronegative oxygen atom. Polar molecules attract one another by dipole-dipole forces, as the positive end of one molecule is attracted to the negative end of the nearby molecule.
What's the difference between polar and dipolar?
Summary – Polar vs Dipolar Molecules The key difference between polar and dipolar molecules is that polar molecules have two opposite ends with opposite electrical charges, whereas dipolar molecules have two poles.
What is meant by dipolar?
Definitions of dipolar. adjective. having equal and opposite electric charges or magnetic poles having opposite signs and separated by a small distance.
Is water polar or dipolar?
2: Water is a polar molecule, as greater electron density is found around the more electronegative oxygen atom. Polar molecules attract one another by dipole-dipole forces, as the positive end of one molecule is attracted to the negative end of the nearby molecule.
Why water can dissolve more substances?
It is water's chemical composition and physical attributes that make it such an excellent solvent. Water molecules have a polar arrangement of oxygen and hydrogen atoms—one side (hydrogen) has a positive electrical charge and the other side (oxygen) had a negative charge.
Can dissolve more substance than any other liquid because?
Answer - Water can dissolve more substance than any other liquid because of its polarity and ability to form hydrogen bonds. A substance that displaces most compounds is known as a universal solvent.
Why does water have a dipole moment?
We can say that water has a dipole moment because it is not a 'balanced' molecule, like C O 2 is. I understand what you are really asking though.
How to tell if a molecule has a dipole moment?
Fundamentally, dipolar simply means that a region of the molecule has a center of positive charge and a center of negative charge.
What does VSEPR say about the geometry of a molecule?
Now, what VSEPR says is that the geometry of the molecule is only decided by the σ bonds and lone pairs on the central atom . You count up the σ bonds and lone pairs (lets say they add up to x ), and decide the geometry based on that. The geometry is the most stable configuration of x hybrid orbitals. In simple terms, if we took x balloons and tied them together, the directions the balloons point in help us correspond to where the bonds and lone pairs lie:
Why do electrons have bent shapes?
These extend outward a greater distance, allowing the electrons to occupy a space that is further away from the other electrons, and therefore requires less energy to do so (because electrons repel each other).
How many electrons are in a water atom?
Now let's take water. The central atom (Oxygen) has a valence configuration of 2 s 2 2 p 4, that is, 6 electrons. In water, since we have two single bonds, we have one σ bond each (and no π bonds). So we have total two σ bonds.
What does the color red mean in electron density?
They add up to give a net dipole moment (shown with grey in the diagram). The colors indicate electron density, red is more dense/blue is less dense . Dipole moment is from low density to high density.
Is water a dipole?
Water ( H X 2 O) is a dipole. The reason why is simply because it is not symmetrical, there are more electrons on the oxygen side than on the hydrogen side, and the electronegativity of oxygen. But why isn't H X 2 O symmetrical like C O X 2?
Why is water polar?
Water ( H 2 O) is polar because of the bent shape of the molecule. The shape means most of the negative charge from the oxygen on side of the molecule and the positive charge of the hydrogen atoms is on the other side of the molecule. This is an example of polar covalent chemical bonding.
How does water interact with other molecules?
The shape of each water molecule influences the way it interacts with other water molecules and with other substances. Water acts as a polar solvent because it can be attracted to either the positive or negative electrical charge on a solute. The slight negative charge near the oxygen atom attracts nearby hydrogen atoms from water or positive-charged regions of other molecules. The slightly positive hydrogen side of each water molecule attracts other oxygen atoms and negatively-charged regions of other molecules. The hydrogen bond between the hydrogen of one water molecule and oxygen of another holds water together and gives it interesting properties, yet hydrogen bonds are not as strong as covalent bonds. While the water molecules are attracted to each other via hydrogen bonding, about 20% of them are free at any given time to interact with other chemical species. This interaction is called hydration or dissolving.
Why is the shape of a molecule nonpolar?
The reason the shape of the molecule isn't linear and nonpolar (e.g., like CO 2) is because of the difference in electronegativity between hydrogen and oxygen.
What is the bent conformation of water?
The bent conformation is a balance between attraction and repulsion. Remember that even though the covalent bond between each hydrogen and oxygen in water is polar, a water molecule is an electrically neutral molecule overall. Each water molecule has 10 protons and 10 electrons, for a net charge of 0.
Which atoms are flexed away from the two filled orbitals of the oxygen molecule?
The electrically positive portions of the molecule (the hydrogen atoms) are flexed away from the two filled orbitals of the oxygen.
Is water a polar molecule?
Anne Marie Helmenstine, Ph.D. Updated May 06, 2019. Water is a polar molecule and also acts as a polar solvent. When a chemical species is said to be "polar," this means that the positive and negative electrical charges are unevenly distributed. The positive charge comes from the atomic nucleus, while the electrons supply the negative charge.
