
BeF 2 is soluble in water due to greater hydration energy of Be 2+ ion as compared to the crystal lattice.The other fluorides are almost insoluble in water. Since on descending the group lattice energy decreases more rapidly than the hydration energy.
Is BeF2 soluble in water?
> BeF2 is soluble in water, w... Option (A) is correct. almost insoluble in water. Since on descending the group lattice energy decreases more rapidly than the hydration energy. Was this answer helpful?
Why is be soluble in water but not other elements?
While moving down the group the hydration energy decreases, so as the size increases the hydration enthalpy decreases. Be is the first element in the second group with least size, so it possesses the highest hydration enthalpy and hence is soluble in water, whereas others are insoluble.
Why is the solubility of lithium fluoride higher than other halides?
Due to small size lattice energy of LiF is greater. High solubility of Berellium fluoride is due to high hydration enthalpy of small Be2+ ion which more than compensates for high lattice enthalpy of BeF2. In case of other halides the reduced hydration enthalpy is just not enough to compensate the lattice enthalpy,hence they are sparingly soluble.
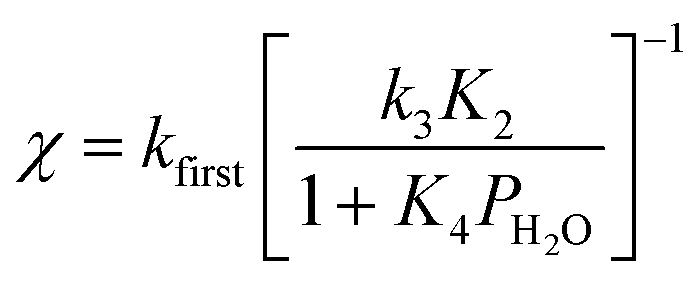
Why BeF2 is more soluble in water?
While moving down the group the hydration energy decreases, so as the size increases the hydration enthalpy decreases. Be is the first element in the second group with least size, so it possesses the highest hydration enthalpy and hence is soluble in water, whereas others are insoluble.
Is BeF2 highly soluble in water?
almost insoluble in water.
Why is BeF2 more soluble in water than BaF2?
Sorry i interpreted the question wrong BaF2 is more soluble than BeF2 because for molecules having small anions like flouride , oxide ,hydroxides etc solubility increases down the group because lattice energy decreases .
Is bf2 soluble in water?
BeF2 is soluble in water whereas fluorides of other alkaline earth metals are insoluble because of. ionic nature of `BeF_2` covalent nature of `BeF_2` hydration energy of `Be^(2+)` ion as compared to its lattice energynone of these.
Is BeF2 ionic or covalent?
In BeF2 molecule Be+2 ion is smaller and also have higher charge on it, hence it will be able to pull electron density away from F-1 ions and introduce the covalence character. That's why BeF2 is a covalent molecule not an ionic.
What is the lattice energy of BeF2?
Lattice energies of BeF2, MgF2, CaF2 and BaF2 are -2906, -2610, -2459 and -2367 KJ/molrespectively.
Which of the following is most soluble BeF2?
BeF2 has highest solubility as Be+2 have high hydration energy.
Why is baf2 soluble in water?
Under standard conditions it adopts the fluorite structure and at high pressure the PbCl2 structure. Like CaF2, it is resilient to and insoluble in water....Barium fluoride.IdentifiersBoiling point2,260 °C (4,100 °F; 2,530 K)Solubility in water1.58 g/L (10 °C) 1.61 g/L (25 °C)Solubility product (Ksp)1.84 × 10−745 more rows
Which of the following is least soluble in water baf2?
Therefore, strontium fluoride has the least solubility.
What is the correct name for BeF2?
BERYLLIUM FLUORIDE Beryllium difluorideBeryllium fluoridePubChem CID24589StructureFind Similar StructuresChemical SafetyLaboratory Chemical Safety Summary (LCSS) DatasheetMolecular FormulaBeF2SynonymsBERYLLIUM FLUORIDE Beryllium difluoride 7787-49-7 beryllium;difluoride Beryllium fluoride(BeF2) More...4 more rows
Is BeF2 more soluble than mgf2?
1 Answer. BeF2 has highest solubility as Be+2 have high hydration energy.
How is BeF2 formed?
Formation of BeF2: When it is in excited state the configuration is 3s22s1,2p1 . (iii) The half filled 2pz orbitals of two fluorine atoms overlap with 'sp' hybrid orbitals of beryllium in their axis to form two (sp – p) bonds. (iv) BeF2 molecule so formed has linear shape.
Is baf2 soluble or insoluble in water?
Barium fluoride (BaF2) is an inorganic compound with the formula BaF2. It is a colorless solid that occurs in nature as the rare mineral frankdicksonite. Under standard conditions it adopts the fluorite structure and at high pressure the PbCl2 structure. Like CaF2, it is resilient to and insoluble in water.
Is BeSO4 soluble in water?
BeSO4 is soluble in water due to high hydration energy of Be2+ ion. The high hydration energy of Be2+ in BeSO4 overcomes the lattice energy factors and therefore BeSO4 is soluble in water.
Which salt is fairly soluble in water?
All sodium, potassium and ammonium salts are soluble, except carbonates. If it's a silver or lead salt, chances are it won't be soluble.
What type of compound is baf2?
Barium fluoride is an ionic compound because it is made up of the barium cation (has a +2 charge) and the fluoride anion (has a -1 charge).