Are the atoms really sharing electrons?
The atoms are not really sharing electrons as much as they are fighting over them this creates a force that makes the atoms stick together
Why is sharing electrons causes atoms to bond together?
- De Leon, Ma. Roselle Angela S. ...
- SHIELA MARI S. CALDERON BEED-IV (Tutorial) A chemical bond is a lasting attraction between atoms, ions or molecules that enables the formation of chemical compounds.
- John Lawrence P. Delos Santos. ...
- Jahna Carmella B. Galvez. ...
- Villaroman, Nikki D.C. BEEd III. ...
- Ma. ...
- Marife DG. ...
- Patrisha Anne D.Vanguardia. ...
- Louise Jerome P. ...
Why does atom gains or loses its electrons?
When accepting an electron stabilizes the atom, then the atom will reach a lower energy state by releasing the energy. This is because, higher the stability, lower is the energy content. So, higher the electron affinity, greater is the stabilization by gaining the electron, and more is the energy released.
Why do atoms give or take away electrons?
Some atoms have too many electrons(one or two extra). These atoms like to give up their electrons. Some atoms are really close to having a full shell. Those atoms go around looking for other atoms who want to give up an electron.
Why do atoms share their electrons?
Atoms share their outermost valence electrons to fill up their outer electron shell and gain stability. Atoms mutually try to share their electrons with each other to complete the Octet Rule. The Octet Rule requires eight electrons to exist and fill their s- and p- orbital, which is termed as a noble gas configuration. The only elements that are likely not to form covalent bonds are potassium (K) and argon (Ar).
Why do atoms share their outermost electron shells?
By sharing their outermost valence electrons, atoms can fill up their outer electron shell and gain stability. Covalent bonding occurs when pairs of electrons are shared by atoms.
What type of bond is polar covalent?
A polar covalent bond is a type of chemical bond where one pair of electrons is shared unevenly between two atoms . Polar covalent bonds are an intermediate situation between ionic bonding and covalent bonding, where one side of the molecule gets negatively charged and the other side of the molecule gets positively charged.
How many covalent bonds can non-metals form?
Non-metals are able to form covalent bonds with other non-metals. They do this by forming anywhere from 1-3 covalent bonds depending on the number of outer-most valence electrons that they possess in the valence shell.
What type of bond is a covalent bond?
The shared pair of electrons then form a new orbit that spreads around the nuclei of both the atoms, building a molecule. There are two types of covalent bonds: polar covalent bonds and hydrogen bonds.
Why do atoms form chemical bonds?
In order to make the outer electron shell more stable, atoms form chemical bonds. A non-polar covalent bond is a covalent bond in which the bonding electrons are shared equally between two atoms. Since electrons are equally shared, it makes it unique.
How do atoms gain stability?
One atom gains stability by losing its outer electrons and the other atom gains stability by filling up its outer shell by gaining electrons. A covalent bond is formed when this sharing of electrons between atoms provides them the highest stability.
How many electrons do atoms need to be stable?
Most of the atoms, have an incomplete last orbit. In order to attain stability, they need to have 8 electrons in the outermost orbit also known as the octet state. Some elements like require 2 electrons in their outer orbit also known as the duplet state. When all the orbitals are completely filled they become stable, and do not take part in further exchange of electrons.
What is another method of separating electrons?
Another method is using electricity itself to separate electrons. This happens in cathode ray tubes (eg. the Old TV sets) and in semiconductors (eg. computer chips).
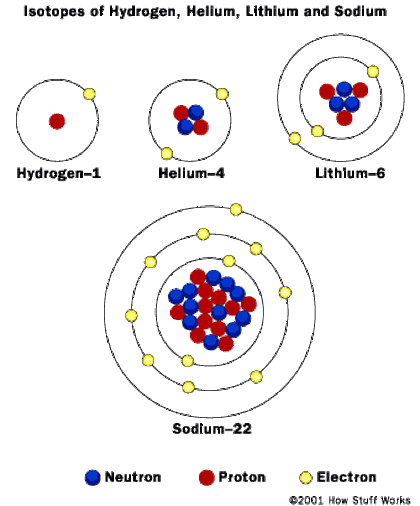
How many electrons are in a hydrogen atom?
In Hydrogen, there is only one orbit so the electron is close to the nucleus and is tightly bound. However, the shell (as it is called) is not full; the inner shell needs 2 electrons. So when two hydrogen atoms come together, the electrons want to orbit both nuclei , and they do, so gaseous hydrogen at normal temperature forms two atoms in a molecule H2.
How to separate electrons at 3000K?
At very high temperatures > 3000K there is a fourth state of matter, a plasma, where the velocity of molecules is so high, that the electrons are knocked off and nuclei and electrons tend to move around independently.
How does static electricity work?
Static electricity is where electrons (from outer orbits) are moved from one piece to another. The shock one gets from static electricity is the electrons moving back to the nucleus and hence a current flows.
What is orbital in quantum mechanics?
First, orbitals are our way of describing what is going on, it's not something that is fundamental. Orbitals are simply a useful approximation, but you could imagine that some alien species of quantum mechanics, would not use the concept of orbitals.
Which group of the periodic table does not have a reaction?
The noble gases found on group 18 of the periodic table, have a completely filled orbitals and thus do not take part in a reaction or exhange of electrons.
Why do atoms gain or lose valence electrons?
When these atoms gain electrons, they acquire a negative charge because they now possess more electrons than protons. Negatively charged ions are called anions.
When do atoms lose electrons?
atoms share ,gain, or lose electrons when chemical bonds form. ionic bonds form when electrons are transferred from one atom to another atom. ions of different elements can combine by forming ionic bonds . positive ions & negative ions form when atom s lose or gain electrons. Similar Asks.
How are ions formed?
Ions are formed when atoms lose or gain electrons in order to fulfill the octet rule and have full outer valence electron shells. When they lose electrons, they become positively charged and are named cations. When they gain electrons, they are negatively charged and are named anions.
What are negatively charged ions called?
Negatively charged ions are called anions . Thereof, how do atoms gain and lose electrons? When atoms lose or gain electrons, they become what are called ions. Loss of electrons leaves an atom with a net positive charge, and the atom is called a cation. Gain of electrons leaves an atom with a net negative charge, and the atom is called an anion.
Why do some elements share electrons?
The atoms of some elements share electrons because this gives them a full valence shell.
What happens when atoms can't reach their full outer shell?
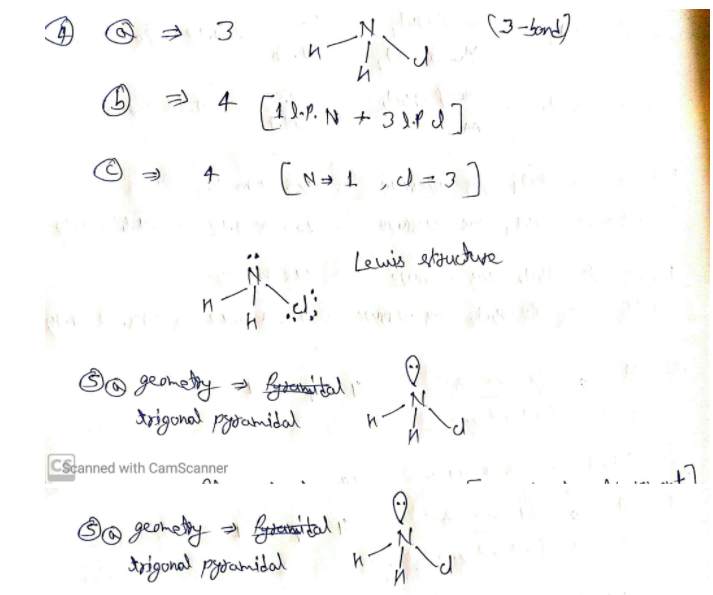
If atoms can’t achieve a full outer shell by transferring electrons, they resort to sharing. In this way, each atom can count the shared electrons as part of its own valence shell. This sharing of electrons is covalent bonding.
How many electrons does an oxygen atom have?
For example, an oxygen atom has six electrons in its valence shell. The most the shell can hold is eight. Two oxygen atoms can share their valence electrons as shown below.
