The function of valence electrons is to transfer between the atom An atom is the smallest constituent unit of ordinary matter that has the properties of a chemical element. Every solid, liquid, gas, and plasma is composed of neutral or ionized atoms. Atoms are extremely small; typical sizes are around 100 picometers (1×10⁻¹⁰ m, a ten-milliont…Atom
Why is the number of valence electrons important?
Meanwhile, the number of valence electrons present also helps us determine a specific element’s chemical properties, such as its valence or valency, the formation of bonds with other elements. It also gives us an idea of how readily the atoms can form bonds, the number of unpaired electrons and how many atoms can take part.
How do you determine the valency of an element?
The valency of an element can be determined by valence electrons. The number of electrons in the last shell is the valence electron of that element. To determine the valence electron must have a good idea about the electron configuration. Valence electrons participate in chemical reactions and bond formation.
What is the meaning of Valence?
Valence is the number of electrons an atom must lose or gain to attain the nearest noble gas or inert gas electronic configuration. “Electrons in the outer shells that are not filled are called valence electrons”.
What is the role of electrons in chemical bonding?
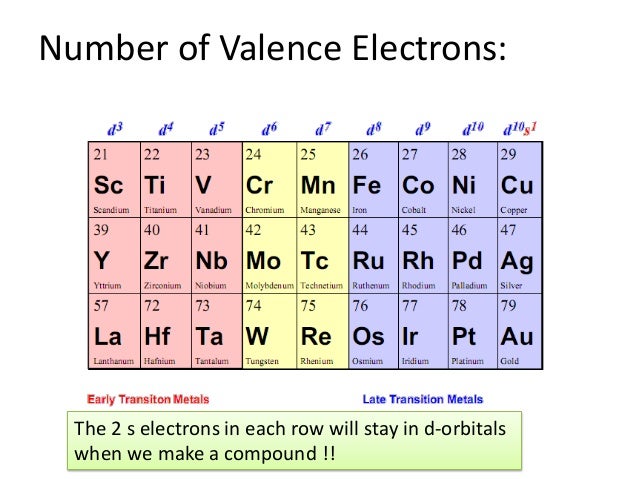
Electrons are involved in the chemical bonding and reactions of the atom. It is said to occupy orbitals in an atom. The number of valence electrons of an atom can be obtained from the periodic table because it is equal to the group number of the atom.
Why are valence electrons important?
They are the electrons that exist in the outermost shell/cloud of an atom at the highest energy level. When atoms interact chemically through bonding , it's their outer shell electrons that are involved. The number of outer shell or valence electrons influences how the element will react by gaining or losing electrons to create stability. The periodic table of elements is arranged to reflect this. Elements with the same number of valence electrons are in the same column. The less valence electrons an atom holds, the less stable it is.
What are the valence electrons?
Valence electrons are quite important. They are the electrons that exist in the outermost shell/cloud of an atom at the highest energy level. When atoms interact chemically through bonding, it's their outer shell electrons that are involved. The number of outer shell or valence electrons influences how the element will...
Which element has an outermost electron configuration of 4s2?
As an example, calcium is element #20. It is in the fourth period in the second column of the s block. Therefore calcium has an outermost electron configuration of 4s^2.
Why are valence electrons important in chemical reactions?
The valence electrons are part of most of the chemical reactions because they contain more energy compared to the electrons present in inner orbits. Meanwhile, the number of valence electrons present also helps us determine a specific element’s chemical properties, such as its valence or valency, the formation of bonds with other elements. It also gives us an idea of how readily the atoms can form bonds, the number of unpaired electrons and how many atoms can take part.
What are Valence Electrons?
Valence is the number of electrons an atom must lose or gain to attain the nearest noble gas or inert gas electronic configuration. “Electrons in the outer shells that are not filled are called valence electrons”.
How many electrons are in the last shell of an orbit?
The total number of electrons present in last shell orbit is known as valence electron. For example: Oxygen have 6 electrons present in last orbital shell hence 6 is valence electron.
How stable are atoms?
Atoms are most stable if they have a filled valence shell of electrons. Atoms transfer or share electrons in such a way that they can attain a filled shell of electrons. For the main group elements, the valence electron exists only in the outermost electron shell. A valence electron can exist in the inner shell of a transition metal.
Why are electrons important in chemistry?
Electrons are involved in the chemical bonding and reactions of the atom. It is said to occupy orbitals in an atom. The number of valence electrons of an atom can be obtained from the periodic table because it is equal to the group number of the atom. Atoms are most stable if they have a filled valence shell of electrons.
How do Lewis structures help us?
Lewis structures help us to track the valence electrons and predict the types of bond. Valence electrons are all arranged in different orbitals or shells and are mostly negatively charged particles. Further, these electrons are responsible for interaction between atoms and the formation of chemical bonds.
What are the s and p electrons in the outermost shell?
Valence electrons are the s and p electrons in the outermost shell. The electrons present in the inner shell are core electrons. When we study and observe the atom of an element, we come across tiny subatomic particles called valence electrons.