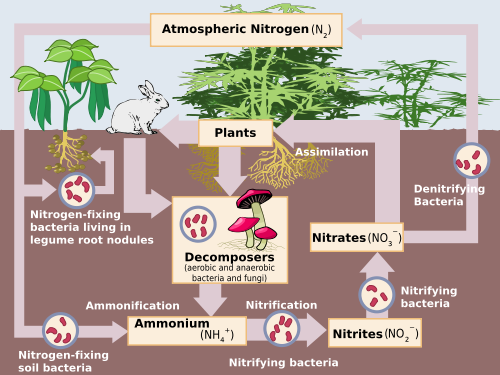
Earth’s atmosphere contains a huge pool of nitrogen gas (N2). But this nitrogen is “unavailable” to plants, because the gaseous form cannot be used directly by plants without undergoing a transformation. To be used by plants, the N2must be transformed through a process called nitrogen fixation. What is the main source of nitrogen?
Why can plants and animals not use atmospheric nitrogen directly?
Why can plants and animals not use atmospheric nitrogen directly? What's the basic reason? They have difficulties changing it onto a form that can be absorbed by root systems, such as ammonia, nitrates or urea. Molecular nitrogen is very stable and takes considerable energy to get to absorbable forms.
What causes nitrogen in the atmosphere?
Lightening causes atmospheric N 2 to be converted into nitrate, while plant decay results in volatile nitrogen release. Fogs in coastal areas are also a source of this gas as far as the atmosphere is concerned. 4% of plant matter’s dry weight is nitrogen. Plants require adequate supply of this element for their proper growth and development.
How do plants absorb nitrogen from the atmosphere?
Plants especially leguminous plants don't absorb atmospheric nitrogen. But nitrogen is necessary for them. So they have a nitrogen fixing bacteria in their roots like Rhizopus, nostoc etc which converts atmospheric nitrogen into amino acids , which are absorbed by plants.
Why don't animals use nitrogen fixed by lightning?
There is actually quite a bit of nitrogen fixed by lightning and specialized micro-organisms that evolution did not support “wasting” plant metabolic resources on nitrogen fixation. Of course, animals get all their nitrogen compounds from plants eventually. No lifeform requires Nitrogen (N2) is the reason. Think about it.

Why is N2 not easily used by plants and animals?
Nitrogen in its gaseous form (N2) can't be used by most living things. (Plants for example, do not have the required enzymes to make use of atmospheric nitrogen.) It has to be converted or 'fixed' to a more usable form through a process called fixation.
Why can't plants use N2 gas?
A plant can not use atmospheric nitrogen directly because it is present in free gaseous form in the atmosphere whereas plants are capable of absorbing N2 in the form of Nitrogen compounds like Nitrites and nitrates only from the soil, which is converted by the microorganisms in the soil.
Can plants and animals use N2?
Nitrogen in the gaseous form cannot be absorbed and used as a nutrient by plants and animals; it must first be converted by nitrifying bacteria, so that it can enter food chains as a part of the nitrogen cycle.
Why nitrogen Cannot be used by animals?
Nitrogen is a building block of all living organisms. Plants and animals cannot absorb nitrogen directly from the air. So they are oxidized into Nitrates and Nitrites by the process of nitrogen fixation and then they are absorbed by the plants. Q.
Can plants use atmospheric nitrogen?
Although the atmosphere is mostly made up of nitrogen, it is in the form of a gas known as dinitrogen N2. Plants cannot use this form. Dinitrogen, or atmospheric nitrogen, can also be found in the soil.
Can plants take in nitrogen from the atmosphere?
Plants cannot themselves obtain their nitrogen from the air but rely mainly on the supply of combined nitrogen in the form of ammonia, or nitrates, resulting from nitrogen fixation by free-living bacteria in the soil or bacteria living symbiotically in nodules on the roots of legumes.
What form of nitrogen is available to plants?
Plant available forms of nitrogen (N) are inorganic and include nitrate (NO3), and ammonium, (NH4). Prior to analysis, soil samples should be air dried rather than oven dried at high temperature (> 30ºC) to prevent N loss through volatilization.
Is nitrogen used by plants or animals?
Nitrogen is needed both by Plants and Animals because it is the major constituent of proteins, vitamins, hormones etc. Nitrogen is a crucially important component of life. It is an abundant element present in the atmosphere.
In what ways is n2 gas removed from the atmosphere?
A small amount of nitrogen is fixed by lightning, but most of the nitrogen harvested from the atmosphere is removed by nitrogen-fixing bacteria and cyanobacteria (formerly called blue-green algae).
What plants Cannot use atmospheric?
Answer. plants can't use atmospheric nitrogen so bacteria present in soil fix nitrates in soul so they can be used by plants.
Which gas Cannot be absorbed by plants directly from air?
Plants cannot utilise the atmospheric nitrogen directly.
Why is nitrogen good for animals?
Nitrogen is essential for all living things because it is a major part of amino acids, which are the building blocks of proteins and of nucleic acids such as DNA, which transfers genetic information to subsequent generations of organisms.
How is nitrogen converted to a form that plants and animals can use?
Nitrogen is converted from atmospheric nitrogen (N2) into usable forms, such as NO2-, in a process known as fixation. The majority of nitrogen is fixed by bacteria, most of which are symbiotic with plants. Recently fixed ammonia is then converted to biologically useful forms by specialized bacteria.
What are 2 ways nitrogen becomes useable to plants humans and animals?
Plant and animal wastes decompose, adding nitrogen to the soil. Bacteria in the soil convert those forms of nitrogen into forms plants can use. Plants use the nitrogen in the soil to grow. People and animals eat the plants; then animal and plant residues return nitrogen to the soil again, completing the cycle.
Can animals use nitrogen directly out of the air?
However, most organisms, including plants, animals and fungi, cannot get the nitrogen they need from the atmospheric supply. They can use only the nitrogen that is already in compound form.
Why do plants need nitrogen?
Plants require adequate supply of this element for their proper growth and development. This is because it plays a crucial role in the formation of compounds like protein, amino acids, nucleic acids, etc. Though 78% of the Earth’s atmosphere imbibes nitrogen, plants and animals have no direct way of accessing it.
What is the role of nitrogen in plants?
Plants require adequate supply of this element for their proper growth and development. This is because it plays a crucial role in the formation of compounds like protein, amino acids, nucleic acids, etc. Though 78% of the Earth’s atmosphere imbibes nitrogen, plants and animals have no direct way of accessing it. Atmospheric nitrogen cannot be absorbed by the plants, instead it has to be fixed or transformed into another biologically suitable compound that can be absorbed by them. This process is called nitrogen fixation or nitrogen cycle. It is also be seen to form 3% of the human body weight, where it is seen to play a crucial role in food digestion and overall body growth. Proteins ingested in the diet contain nitrogen, which after metabolism is used to form adenosine triphosphate (ATP) molecules. ATP molecules are energy sources for the body.
Why is nitrogen important to life?
Nitrogen in the atmosphere is more abundant than life-sustaining oxygen. This gas is needed by humans, animals, and plants for manufacturing proteins and other essential building units.
What is the triple bond between two nitrogen atoms?
Non-metal nitrogen consists of five electrons in its outermost shell, thereby forming triple bonds in most compounds. Thus, a triple bond is formed between two nit rogen atoms ...
What is the nitrogen fixation process?
It is also be seen to form 3% of the human body weight, where it is seen to play a crucial role in food digestion and overall body growth. Proteins ingested in the diet contain nitrogen, which after metabolism is used to form adenosine triphosphate (ATP) molecules.
How abundant is nitrogen?
Get in touch with us and we'll talk... Let's Work Together! As compared to oxygen, nitrogen is four times more abundant in the Earth’s atmosphere (78.084%). Moreover, it is also known as the seventh most abundant element found in the universe.
Where is nitrogen found?
Nitrogen is also found in all living organisms. In fact, it is like carbon and is indispensable for survival of life on Earth. Nitrogen is also found in large amounts in animal wastes in the form of uric acid, urea, ammonia, etc. Cottonseed meals, linseed meals, etc., are also sources of this molecule. Lightening causes atmospheric N 2 to be converted into nitrate, while plant decay results in volatile nitrogen release. Fogs in coastal areas are also a source of this gas as far as the atmosphere is concerned.
Answer
During nitrogen cycle, nitrogen gas from the atmosphere is converted into ammonia by nitrogen-fixing bacteria, which live in the soil or water. Examples of these are nitrobacter and nitrosomonasspecies (azobacter). These bacteria convert nitrogen to nitrites and nitrates.
Answer
Since plants cannot make use of nitrogen in this form, they need the help of some organisms which are Bacteria; nitrifying bacteria etc o help in the conversion of this form of nitrogen to that particular form mostly nitrates they can utilize directly.
New questions in Chemistry
Millions of years ago in many different regions of the world, large-scale burials of organic matter were followed by the formation of underground trap …
