How do you find the boiling point of ethanol? The normal boiling point for ethanol is 78.4° C and the boiling point elevation is 1.22° C per molal. So the boiling point will be elevation by 8.25 * 1.22° C = 10.1° C. So the boiling point will be 78.4+ 10.1 = 88.5° C.
Full Answer
What is theoretical value of ethanol boiling point?
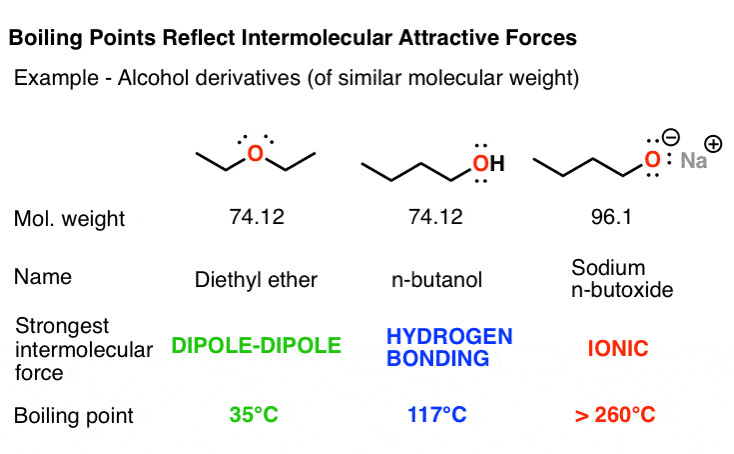
stronger hydrogen bonds and make alcohols gaseous, so alchohol have higher boiling points. 4. The theoretical value of ethanol’s boiling point is 78.3°C.The experimental value of boiling point is 78°C.
Why does ethanol have higher boiling point than acetone?
The higher boiling point for ethanol is observed due to the OH structure that causes hydrogen bonding between the molecules. Acetone has a polar CO double bond, which results in dipole-dipole forces. Since hydrogen bonding is stronger than dipole-dipole forces, ethanol has a higher boiling point. Is acetone more flammable than alcohol?
What is the boiling point of ethanol in degrees Fahrenheit?
The boiling points of alcohols of equal molecular weights are much higher than those of alkanes. Ethanol, for example, has a boiling point of 78 °C (173 °F) with a molecular weight (MW) of 46, while propane (MW 44) has a boiling point of −42 °C (−44 °F).
What is the melting and boiling point of ethanol?
What is the melting and boiling point of alcohol? The boiling points of alcohols are much higher than those of alkanes with similar molecular weights. For example, ethanol, with a molecular weight (MW) of 46, has a boiling point of 78 °C (173 °F), whereas propane (MW 44) has a boiling point of −42 °C (−44 °F).
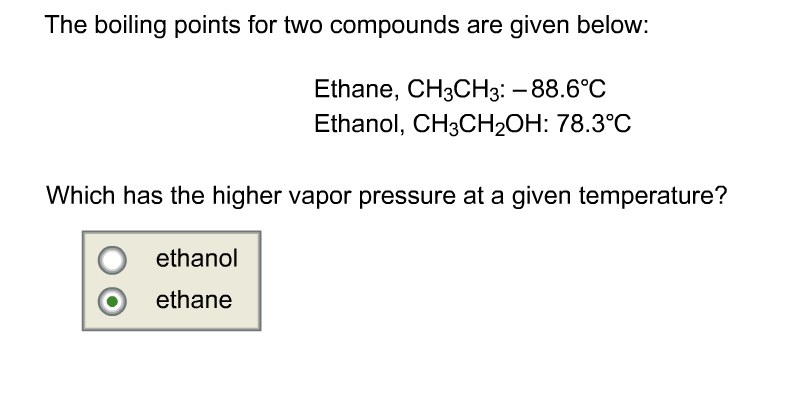
What is the boiling point of ethanol?
173.1°F (78.37°C)Ethanol / Boiling point
How do you determine the boiling point of alcohol?
The boiling point of ethanol or grain alcohol (C2H5OH) at atmospheric pressure (14.7 psia, 1 bar absolute) is 173.1 F (78.37 C).
How do you determine the boiling point?
The temperature at which a pure organic substance changes from the liquid phase to the gas phase is known as the boiling point. A liquid's boiling point can be determined using the capillary method, where an inverted capillary is placed in the liquid of interest and the liquid is heated.
How do you find the boiling point of an aqueous solution?
Example: Calculating Boiling and Freezing Point of an Electrolyte SolutionCalculate the boiling point elevation: ΔTb = Kbm. Kb = 0.512 (from table above) Since: NaCl → Na+(aq) + Cl-(aq): ... Calculate the boiling point of the solution: Tb (solution) = normal boiling point + ΔTb Tb (solution) = 100.00 + 0.154 = 100.154oC.
What is the boiling point of 95% ethanol?
78.5°C.Boiling Point/Range 78.5°C.
What are the boiling points for water and ethanol?
In the case of mixtures of ethanol and water, this minimum occurs with 95.6% by mass of ethanol in the mixture. The boiling point of this mixture is 78.2°C, compared with the boiling point of pure ethanol at 78.5°C, and water at 100°C.
How do you determine boiling point and melting point?
3:595:43Determination of the Melting and Boiling Points of CompoundsYouTubeStart of suggested clipEnd of suggested clipPlace the sample and the thermometer assembly into an oil bath. Hit the oil bath gently observe theMorePlace the sample and the thermometer assembly into an oil bath. Hit the oil bath gently observe the change on a sample until the solid sample melts completely recorder mountain range of a sample.
How do you find boiling point with temperature and pressure?
6:1611:11Vapor Pressure - Normal Boiling Point & Clausius Clapeyron EquationYouTubeStart of suggested clipEnd of suggested clipThe normal boiling point of a liquid is the temperature. At which the vapor pressure of the liquid.MoreThe normal boiling point of a liquid is the temperature. At which the vapor pressure of the liquid. Is equal to the atmospheric pressure.
What determines boiling point of a substance?
Boiling. A liquid boils at a temperature at which its vapor pressure is equal to the pressure of the gas above it. The lower the pressure of a gas above a liquid, the lower the temperature at which the liquid will boil.
How do you determine boiling point and melting point?
3:595:43Determination of the Melting and Boiling Points of CompoundsYouTubeStart of suggested clipEnd of suggested clipPlace the sample and the thermometer assembly into an oil bath. Hit the oil bath gently observe theMorePlace the sample and the thermometer assembly into an oil bath. Hit the oil bath gently observe the change on a sample until the solid sample melts completely recorder mountain range of a sample.
Which alcohol would have a higher boiling point?
*Ph represents the phenyl group, C6H5—. The boiling points of alcohols are much higher than those of alkanes with similar molecular weights. For example, ethanol, with a molecular weight (MW) of 46, has a boiling point of 78 °C (173 °F), whereas propane (MW 44) has a boiling point of −42 °C (−44 °F).
How do you determine the boiling point of an organic compound?
1. Keep the lower end of the ignition tube and the thermometer bulb at the same level. 2. Record the temperature as the boiling point at which brisk and continuous evolution of the bubbles starts from the lower end of the capillary dipped in the liquid organic compound.
What does the boiling point of alcohol depend on?
The boiling point of alcohol depends on which type of alcohol you're using, as well as the atmospheric pressure. The boiling point decreases as atmospheric pressure decreases, so it will be slightly lower unless you are at sea level. Here is a look at the boiling point of different types of alcohol.
What is the boiling point of water?
Distillation may also be used to separate alcohol and water. The boiling point of water is 212 F or 100 C , which is higher than that of alcohol. However, distillation can't be used to fully separate the two chemicals.
How to separate alcohol from water?
One practical application of the different boiling points of alcohols and of alcohol with respect to water and other liquids is that it can be used to separate them using distillation. In the process of distillation , a liquid is carefully heated so more volatile compounds boil away. They may be collected, as a method of distilling alcohol, or the method may be used to purify the original liquid by removing compounds with a lower boiling point. Different types of alcohol have different boiling points, so this can be used to separate them from each other and from other organic compounds. Distillation may also be used to separate alcohol and water. The boiling point of water is 212 F or 100 C, which is higher than that of alcohol. However, distillation can't be used to fully separate the two chemicals.
How to remove alcohol from a liquid?
The only way to completely remove alcohol from a liquid is to boil it away completely or allow it to evaporate until it's dry . Helmenstine, Anne Marie, Ph.D. "Boiling Points of Ethanol, Methanol, and Isopropyl Alcohol.". ThoughtCo, Aug. 26, 2020, thoughtco.com/boiling-point-of-alcohol-608491.
How long does it take to get alcohol out of a recipe?
A recipe had to be baked 2 hours or longer to bring the alcohol content down to 10 percent or lower.
How much retention does flaming alcohol have?
Flaming the liquid to burn off the alcohol still allowed for 75 percent retention.
Does alcohol leave water in cooking?
While it makes sense cooking food above 173 F or 78 C would drive off the alcohol and leave the water, scientists at the University of Idaho Department of Agriculture have measured the amount of alcohol remaining in foods and found most cooking methods don't actually affect the alcohol content as much as you might think .
What is the boiling point of a liquid?
Boiling point is a temperature at which the pressure exerted by the surroundings upon a liquid equals to the pressure exerted by the vapor of the liquid. At this point, addition of heat results in the transformation of the liquid into its vapor without raising the temperature.
What would be the boiling point of a substance if it was not in a solution state?
If the substance was not in a solution state, then 0.0238 degrees celsius would have been its boiling point. Since water is also present, we add the boiling point of water which 100 degrees celsius to 0.0238 degrees celsius to get 100.0238 degrees celsius.