How many atoms are in an amino group?
What are the elements that make up the periodic table?
What are the components of proteins?
What are the properties of carbon?
Where is hydrogen found in the body?
Where is phosphorus found?
Is nitrogen a gas?
See 4 more
About this website

Are there atoms in every living thing?
All living things are based on atoms and their interactions.
How many atoms are in every human?
6,500,000,000,000,000,000,000,000,000Suzanne Bell, an analytical chemist at West Virginia University, estimates that a 150-pound human body contains about 6.5 octillion (that's 6,500,000,000,000,000,000,000,000,000) atoms. The vast majority of them are hydrogen (humans are almost entirely water, which comprises two hydrogen atoms and an oxygen).
How many atoms are in a baby?
In summary, for a typical human of 70 kg, there are almost 7*1027 atoms (that's a 7 followed by 27 zeros!) Another way of saying this is "seven billion billion billion." Of this, almost 2/3 is hydrogen, 1/4 is oxygen, and about 1/10 is carbon.
How many atoms are in a galaxy?
Our galaxy, the Milky Way, contains approximately 100 to 400 billion stars. If we take this as 200 billion or 2 × 1011 stars and assume that our sun is a reasonable average size we can calculate that our galaxy contains about (1.2 × 1056) × (2 × 1011) = 2.4 × 1067 atoms.
Where were my atoms before I was born?
The particles we're made of The hydrogen atoms in you were produced in the big bang, and the carbon, nitrogen and oxygen atoms were made in burning stars. The very heavy elements in you were made in exploding stars.
Can atoms be destroyed?
According to the law of conservation of energy, the matter cannot be created nor be destroyed. Hence, an atom cannot be destroyed and it cannot be broken into smaller particles.
How many atoms are in a breath of air?
The average adult human tidal volume (volume of breath during rest) is 0.5 liters. At Standard Temperature and Pressure (STP) 1 mole of air (6.022*1023 atoms) occupies 22.4 liters. This would equal around 1.344*1022 atoms in an average breath.
How big is a atom?
Atoms and Sub-Atomic Particles **Size: **Atoms have an average radius of about 0.1 nm. About 5 million hydrogen atoms could fit into a pin head. The nucleus of an atom is 10,000 times smaller than the atom. If an atom was the size of Wembley Stadium, then the nucleus would be the size of a garden pea.
How many atoms are in an apple?
By the same estimate, an apple is about 10^9 angstroms (7–8.5cm, pick the size you like) which gives a volume of 10^27 cubic angstroms. So… 10^27 atoms in an apple … ball-park.
Are there infinite atoms?
But on the cosmic scale of the universe, we can assume that the amount of matter created and uncreated cancel each other out. This means matter is finite, so there are the same number of atoms in the observable universe as there always have been, according to Scientific American.
What's bigger than the universe?
No, the universe contains all solar systems, and galaxies. Our Sun is just one star among the hundreds of billions of stars in our Milky Way Galaxy, and the universe is made up of all the galaxies – billions of them.
Is our universe an atom?
In fact, the Universe we see through our telescopes may be just the collection of billions of atoms that are in a larger Universe. Perhaps we are even part of the atoms on another gigantic living being!
How many atoms are in a pencil?
How many atoms are in the leads of 12 lead pencils? Pencil leads are made of carbon atoms. Carbon has an atomic mass of 12. Atomic mass is the mass of an atom....ExponentValueAnalogy1023100 000 000 000 000 000 000 000The entire surface of the earth plus 99 planets just like earth covered 1 m deep with peas15 more rows
Is virus made of atoms?
Viruses are made up of only atoms and molecules; they contain genes in the form of either DNA or RNA. They have no metabolic activity and therefore, cannot maintain a steady internal state. When a virus infects cells, it forces the cell to make more copies of the virus.
How many atoms are in a breath of air?
The average adult human tidal volume (volume of breath during rest) is 0.5 liters. At Standard Temperature and Pressure (STP) 1 mole of air (6.022*1023 atoms) occupies 22.4 liters. This would equal around 1.344*1022 atoms in an average breath.
What is smaller than an atom?
Particles that are smaller than the atom are called subatomic particles. The three main subatomic particles that form an atom are protons, neutrons, and electrons.
List the six elements that are most important to all life - CHNOPS
Study with Quizlet and memorize flashcards containing terms like What type of elements they are, What percentage of the human body (and all other organisms) is made up of these elements, The 6 elements and more.
Top 10 Most Common Earth Elements - V.M. Simandan
The earth is full of different elements that benefit us every day. We may not even consider them to be important to us yet they help sustain life on earth.
How many atoms are in an amino group?
In chemical terms, an amino group consists of one nitrogen atom and two hydrogen atoms. While protein is often thought of mainly as a dietary component, proteins are the drivers of everyday life, catalyzing essential biochemical reactions that build the organs and tissues that keep living things growing, adapting and reproducing.
What are the elements that make up the periodic table?
Six elements on the periodic table account for 97 percent of your body's mass: carbon, hydrogen, nitrogen, oxygen, sulfur and phosphorus. Not coincidentally, these elements exist in great abundance in the Milky Way galaxy and beyond. Human beings are, as a popular saying suggests, stardust. The names of these six elements can be remembered using ...
What are the components of proteins?
Carbon is a major component of amino acids, the building blocks of proteins. Proteins, in turn, make up the structural components of most organs and tissues, including muscle, enzymes and neurons. Brought to you by Sciencing. Brought to you by Sciencing.
What are the properties of carbon?
Carbon's ubiquitous nature on Earth and beyond lies in its ability to form different types of chemical bonds: single, double and triple. With this property, carbon can join with a wide range of other elements. Carbon is a major component of amino acids, the building blocks of proteins. Proteins, in turn, make up the structural components of most organs and tissues, including muscle, enzymes and neurons.
Where is hydrogen found in the body?
In addition, the starchy components of plants that give them their shape are made up of carbohydrates. Water, which makes up more than two-thirds of the human body, contains hydrogen.
Where is phosphorus found?
Phosphorus is also found in bone, and chemical energy derived from metabolic processes is stored for immediate use in phosphorus-based compounds such as ADP (adenosine diphosphate) and ATP (adenosine diphosphate).
Is nitrogen a gas?
Although nitrogen may get comparatively little attention, it is abundant in nature. More than three-fourths of the Earth's atmosphere consists of nitrogen gas. Nitrogen is found in all amino acids and thus in all proteins. In chemical terms, an amino group consists of one nitrogen atom and two hydrogen atoms.
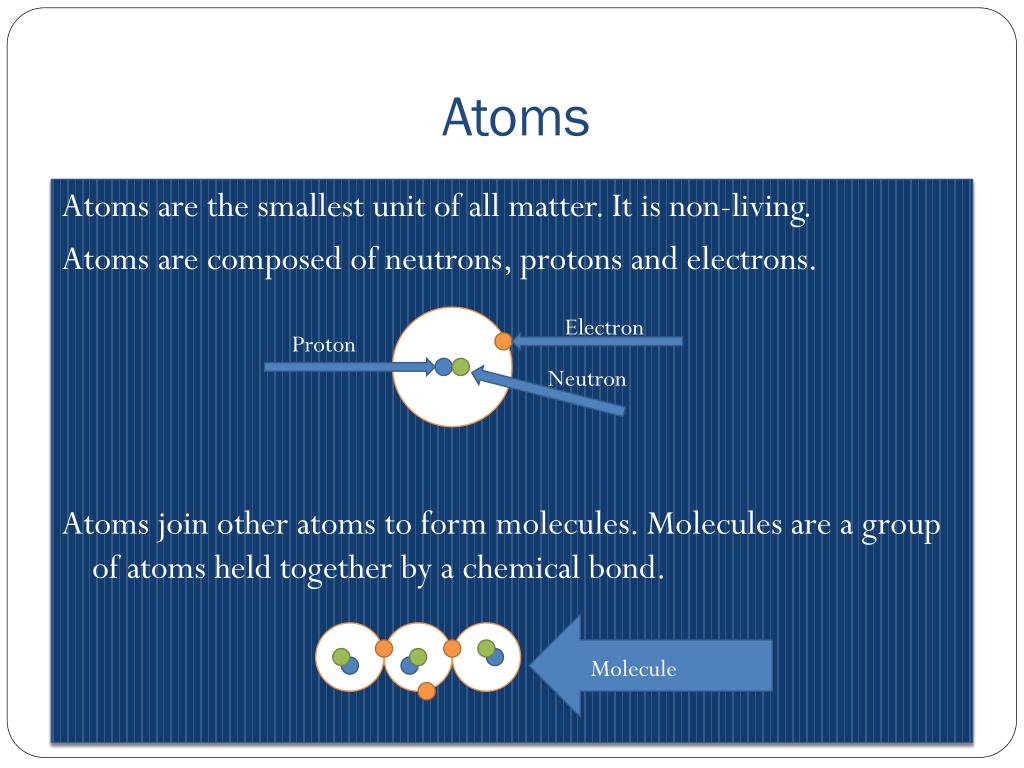
What is an atom?
So now you know the definition of an atom...but that's far from having knowledge of an atom! Atoms are extremely important structures that make up all of the materials on earth. Atoms are in our bodies and they bond together to form molecules, which make up matter.
Why are atoms important?
In the most simple terms, without atoms there would not be a functioning world. Atoms make up matter, and matter makes up everything in the world, with a few exceptions.
How do atoms bond?
The atraction that keeps atoms bonded to each other is called chemical bonding .Chemical bonding keeps atoms together by the postive charge from one atom and the negative charge from another forcing together. The positive charge comes ...
What are the three main forms of matter?
The three main forms of matter are solid, liquid, or gas. The Atomic Theory. In the history of atoms we will go more in depth about Dalton's atomic theory. But as an introduction, the Atomic Theory was a theory that justified the idea that all matter is made up of atoms. This means that mostly anything in the world is made up of atoms.
Why do nonmetal atoms receive valence electrons from the metal atom?
nonmetal atom receives valence electrons from the metal atom due to the transportation of valence electrons. Ionic bonds dissolve in
Which particles do not have atoms?
But it we take microscopic scale this is not true. Elections , light waves, quarks, neutron , protons, etc are many such examples which do not have atoms as their constituent particles.
Why do we consider ourselves living?
We consider ourselves living because , according to us , living things can move themselves , work themselves , think themselves , LIVING THINGS ARE FREE , we are not bonded to anyone's commands or orders , or can move and move stones ourselves , stone doesn't moves us.
What is the basic unit of an object?
Actually atom is the basic or fundamental unit of objects. Smallest particle which object is made of.
Why do non-living things grow?
Non-living things do not show any growth like living ones; their increase in size is always due to addition of more particles from outside.
Do living things have a life cycle?
Living organisms have a definite Life Cycle, whereas, Non-Living objects have no definite Life Cycle.
Do living things have metabolism?
Living things show the process of metabolism. Metabolism is absent in case of Non-living objects.
Do living things need nutrition?
Living things (or organisms) require nutrition, i.e., nourishment and feeding. Non-living things do not require nutrition.
What does the atomic number tell you?
The atomic number tells you the number of electrons in the nucleus.
Which particle is the largest particle in an atom?
Electrons are the largest particle in an atom.
What is the core of an atom made of?
the core of the atom, made up of protons and neutrons
How many atoms are in an amino group?
In chemical terms, an amino group consists of one nitrogen atom and two hydrogen atoms. While protein is often thought of mainly as a dietary component, proteins are the drivers of everyday life, catalyzing essential biochemical reactions that build the organs and tissues that keep living things growing, adapting and reproducing.
What are the elements that make up the periodic table?
Six elements on the periodic table account for 97 percent of your body's mass: carbon, hydrogen, nitrogen, oxygen, sulfur and phosphorus. Not coincidentally, these elements exist in great abundance in the Milky Way galaxy and beyond. Human beings are, as a popular saying suggests, stardust. The names of these six elements can be remembered using ...
What are the components of proteins?
Carbon is a major component of amino acids, the building blocks of proteins. Proteins, in turn, make up the structural components of most organs and tissues, including muscle, enzymes and neurons. Brought to you by Sciencing. Brought to you by Sciencing.
What are the properties of carbon?
Carbon's ubiquitous nature on Earth and beyond lies in its ability to form different types of chemical bonds: single, double and triple. With this property, carbon can join with a wide range of other elements. Carbon is a major component of amino acids, the building blocks of proteins. Proteins, in turn, make up the structural components of most organs and tissues, including muscle, enzymes and neurons.
Where is hydrogen found in the body?
In addition, the starchy components of plants that give them their shape are made up of carbohydrates. Water, which makes up more than two-thirds of the human body, contains hydrogen.
Where is phosphorus found?
Phosphorus is also found in bone, and chemical energy derived from metabolic processes is stored for immediate use in phosphorus-based compounds such as ADP (adenosine diphosphate) and ATP (adenosine diphosphate).
Is nitrogen a gas?
Although nitrogen may get comparatively little attention, it is abundant in nature. More than three-fourths of the Earth's atmosphere consists of nitrogen gas. Nitrogen is found in all amino acids and thus in all proteins. In chemical terms, an amino group consists of one nitrogen atom and two hydrogen atoms.