
Is ice heavier or lighter than liquid water?
Ice floats because it is about 9% less dense than liquid water. In other words, ice takes up about 9% more space than water, so a liter of ice weighs less than liter water. The heavier water displaces the lighter ice, so ice floats to the top.
Does ice have more mass than liquid water?
Twitter. If a person compares the same amount of ice and water, ice does not have more mass than liquid water. However, the volume of the ice is greater than that of liquid water. A reason for this occurring involves the molecular structure of these two different states of water. The bonding of liquid water involves hydrogen bonds attaching ...
What happens if ice was more dense than water?
what would happen if ice was more dense than water || Answer:It would alter ecosystems.Explanation:If ice were to sink and liquid water were to float on top of ice, then that means that all ice would deposit at the bottom of the ocean. This means that the temperatures …
How dense is ice compared to water?
This is owing to the fact that ice has a lower density than liquid water. The density of ice falls by around 9% as it freezes. Because the lattice pattern permits water molecules to spread out more than in a liquid, ice is less dense than water.
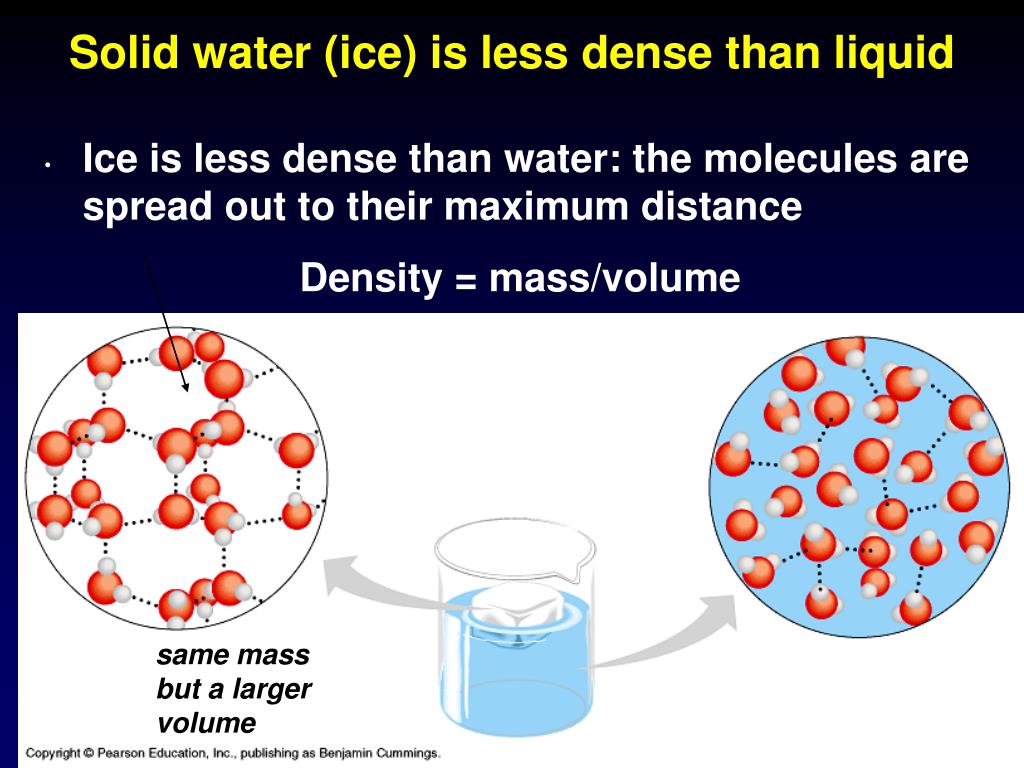
Is liquid water or ice more dense?
The "stuff" (molecules) in water is more tightly packed than in ice, so water has greater density than ice. Don't let the fact that ice is a solid fool you! As water freezes it expands. So, ice has more volume (it takes up more space, but has less density) than water.
Why is ice less dense than liquid water?
When water cools, the hydrogen bonds adjust to hold the negatively-charged oxygen atoms apart, which prevents the ice from becoming any denser. So for water, the density actually decreases along with a decrease in temperature - causing ice to be less dense than water!
Is liquid water the most dense?
As water cools it becomes more dense. At 39°F (or 3.98°C to be exact) water is the most dense. This is because the molecules are closest together at this temperature.
What liquid is denser than water?
Glycerol (or Glycerin) is more dense than water (1.26 g/cc). One could argue that glass is a very slow-moving, viscous liquid (although it has lots of properties of a solid, like rigidity). It's more dense than water. Even saltwater is more dense than water.
Why do solid water float in liquid water?
The mass per unit volume of a substance is called density (density = mass/volume). As the volume of a substance increases, its density decreases. Ice floats because it is less dense than water. Water has a density of 1.0 gm/cubic cm.
What's the most dense form of water?
Water's density is greatest at about 4 °C (39.2 °F), in the liquid phase. Ice, water's solid phase, is more buoyant, so it forms at the surface of water bodies and freezes downward.
What liquid is the heaviest?
MercuryMercury is the heaviest liquid.
Why is water densest in liquid form?
Each water molecule is made up of two hydrogen (H) atoms and one oxygen (O) atom. The bonds between water molecules are called hydrogen bonds. As water cools to 3.98°C, its mass stays the same but volume decreases – the same mass fits into a smaller space so it is more compact.
Why is ice less dense than liquid water quizlet?
Ice is less dense than water because the orientation of hydrogen bonds causes molecules to push farther apart, which lowers the density. More organized in the solid forms verse the liquid form.
Why is ice heavier than water?
Having a lower density means that ice floats when placed in liquid water. When water freezes, it occupies more space than in its liquid form because its molecules expand. Therefore, if we have 1 liter of ice and 1 liter of water, the water will weigh more because it is denser.
Why does ice have a lower density than water?
Since the direction of hydrogen bonds allows molecules to migrate further apart, ice has a lower density than water. Other liquids solidify as the temperature drops so their kinetic energy decreases, allowing molecules to compact more closely and making the solid denser than its liquid nature.
Why does water weigh more than ice?
If we put the same amount of water and ice in the same bottle, water would weigh more than ice. The explanation for this is that water is denser than ice and takes up less volume as compared to ice.
What if scenario: if ice was denser than water?
As the temperature is reduced , water becomes denser until it hits its full density at 4° C. Water has the unusual property of being less dense when the temperature drops from 4° C until it freezes at 0° C. Many marine life types are able to withstand the winter as a result of this.
What are the properties of ice?
What about “heavy ice”? 1 When water is heated, the hydrogen bonds are totally broken by kinetic energy, allowing water molecules to escape into the air as methane (steam or water vapor). 2 As water freezes, the water molecules create a crystalline framework that is held together by hydrogen bonds. 3 Solid water, also known as salt, has a lower density than liquid water. 4 Since the direction of hydrogen bonds allows molecules to migrate further apart, ice has a lower density than water. 5 Other liquids solidify as the temperature drops so their kinetic energy decreases, allowing molecules to compact more closely and making the solid denser than its liquid nature.
What happens to water when it is heated?
When water is heated, the hydrogen bonds are totally broken by kinetic energy, allowing water molecules to escape into the air as methane (steam or water vapor). As water freezes, the water molecules create a crystalline framework that is held together by hydrogen bonds. Solid water, also known as salt, has a lower density than liquid water.
How many hydrogen bonds are there in water?
Hydrogen bonds bind each water molecule to approximately 3.4 other water molecules in liquid water. As water freezes into ice, it crystallizes into a solid lattice, increasing the distance between molecules, with each molecule hydrogen bound to four others.
Why does ice float on water?
As a result, ice floats on water when its mass is lower than that of water.
Why is water denser than ice?
water is denser because there is a little amount of gas dissolved in it but ice is lighter and floats over water water because its density is less than water due to vacant sites that are present in ice molecules, these sites reduce the density of ice.. 336 views. Sponsored by Fx Air.
Which liquid has a density greater than water?
There are many liquids with density greater than water roon temperature. Mercury is denser than water at room temperature and also immiscible with it. Other liquids like honey, milk, crude oil, glycerin, molasses, corn syrup, etc also have a density greater than water at room temperature. 3.4K views. Related Answer.
How does water increase volume?
In ice the molecules of water are bonded through hydrogen bonding . As a result of which a lot of cavities are created in this structure of ice . And hence volume increases and since density is inversely proportional to volume , density decreases . In water there are no such cavities so it's density is greater .
Why is H2O more dense than solid?
Thus, H2O (l) is more dense because of the hydrogen bonding when comparing to its' solid state.
How many hydrogen atoms are in water?
Water molecules are made of 2 hydrogen atoms bonded to 1 oxygen atom. The water molecules are loosely held together with H bonds which means that it takes energy to pull the molecules away from each other i.e., it takes a lot of energy to raise the temperature of water from 0 deg C to 100 deg C. This makes water a liquid at room temperature.
Why does ice float?
Water from liquid to solid ice yields a product less dense, this is why the ice floats. The molecular reasoning behind this is because of Hydrogen Bonding. The partial negative on the oxygen (electronegativity) attracts the partially positive hydrogen in the liquid state.
How much does liquid water weigh?
Unfortunately, liquid water weighs 1,000 kilograms per meter cubed. One of the densest gasses we know of is two orders of magnitude less dense than liquid water.

The Hydrogen Bonds
What About “Heavy Ice”?
- We already mentioned that ice floats on water when it is less solid, yet some types of ice may be denser than ordinary water. Since ice is formed from “heavy water,” it is 10.6 percent denser than regular water. Heavy vapor, D2O instead of H2O, is water in which all hydrogen atoms have been substituted by deuterium, a hydrogen isotope with one proton and one neutron. Strong water is i…
What If Scenario: If Ice Was Denser Than Water?
- As the temperature is reduced, water becomes denser until it hits its full density at 4° C. Water has the unusual property of being less dense when the temperature drops from 4° C until it freezes at 0° C. Many marine life types are able to withstand the winter as a result of this. Although ice is denser than water, it would freeze and fall repeate...