Certain GPCRs have been shown to bind steroid hormones, for example, G protein-coupled estrogen receptor 1 (GPER1) binds estrogen in humans, and Drosophila dopamine/ecdysteroid receptor (DopEcR) binds the molting hormone 20-hydroxyecdysone (20E) in insects.
How are GPCR receptors activated?
For the vast majority of other GPCRs, activation occurs when an agonist diffuses into an unliganded receptor. In many cases the unliganded receptor has some basal (constitutive) activity towards a G protein.
What happens when an agonist binds to a GPCR?
When an agonist binds to a GPCR, the receptor undergoes a conformational shift that is conveyed to the bound G subunit of the heterotrimeric G protein through protein domain dynamics. The activated G subunit exchanges GTP for GDP, resulting in the G subunit's separation from the G dimer and from the receptor.
Do GPCRs transmit animal steroid hormone signals?
1 Shandong Provincial Key Laboratory of Animal Cells and Developmental Biology, School of Life Sciences, Shandong University, Qingdao, 266237, China. [email protected]. G protein-coupled receptors (GPCRs) are cell membrane receptors for various ligands. Recent studies have suggested that GPCRs transmit animal steroid hormone signals.
What is the structure of a GPCR?
G protein-coupled receptor. A GPCR is made up of a long protein that has three basic regions: an extracellular portion (the N-terminus), an intracellular portion (the C-terminus), and a middle segment containing seven transmembrane domains. Beginning at the N-terminus, this long protein winds up and down through the cell membrane,...
See more

What hormones activate G proteins?
Peptide hormones act as ligands for a wide range of G protein-coupled receptors. Peptide hormones are secreted and function in an endocrine manner to regulate many physiological functions, including growth, appetite and energy metabolism, cardiac function, stress, and reproductive physiology.
What does GPCR interact with?
G proteinsWhat Do GPCRs Do? As their name implies, GPCRs interact with G proteins in the plasma membrane. When an external signaling molecule binds to a GPCR, it causes a conformational change in the GPCR. This change then triggers the interaction between the GPCR and a nearby G protein.
What do GPCRs directly bind?
G protein–coupled receptors (GPCRs) mediate the majority of cellular responses to external stimuli. Upon activation by a ligand, the receptor binds to a partner heterotrimeric G protein and promotes exchange of GTP for GDP, leading to dissociation of the G protein into α and βγ subunits that mediate downstream signals.
Does oxytocin bind to a GPCR?
Oxytocin mediates its anxiolytic effect, at least in part, via binding to its GPCR in the hypothalamic paraventricular nucleus, followed by transactivation of the epidermal growth factor receptor, and subsequent activation of a MEK-extracellular signal-regulated kinase (ERK) MAP kinase pathway.
How does a GPCR respond to a ligand?
Upon activation by a ligand, the GPCR undergoes a conformational change and then activate the G proteins by promoting the exchange of GDP/GTP associated with the Gα subunit. This leads to the dissociation of Gβ/Gγ dimer from Gα.
What do GPCRs transport?
GPCRs or G protein-coupled receptors are proteins located on the cell surface that recognize extracellular substances and transmit signals across the cell membrane. GPCRs do this by activating guanine nucleotide-binding proteins (G protein) that are responsible for signal transduction inside the cell.
Is GPCR and enzyme linked receptor?
Enzyme-linked receptors are one of 3 classes of cell surface receptors (the other 2 are ion channel coupled and G-protein coupled receptors (GPCRs)). They are single-span transmembrane proteins and their cytosolic domain has intrinsic enzymatic activity or is associated with an enzyme.
What is GPCR antagonist?
An agonist is a ligand that can stimulate (agonize) the GPCR to activate intracellular signaling and trigger a biological response. In contrast, an antagonist is one such ligand that can inhibit (antagonize) the action of an agonist, either natural or synthetic, to suppress the signaling and biological response.
How many drugs are approved for GPCR?
As of 2020, over 1500 drugs were approved by the FDA, where 460 drugs (36%) target mainly GPCRs. Presently, class A GPCR is targeted by most of the drugs (94%), followed by class B (4%), class C (2%), and class F (2%). Rask et al. reported that around 19% of the human genomes are targeted by drugs, which directly or indirectly affect GPCR (Rask-Andersen et al., 2011). Due to the diverse druggable properties of GPCR, the data showed most of the clinical trials were conducted by the US FDA and other countries for the clinical development of drugs. GPCRs are connected to numerous signaling cascade pathways, exhibiting their effect in minute concentrations (Table 1).
How many GRKs are there?
There are seven GRKs categorized into three subfamilies depending upon sequence and structural similarity. The first subfamily is rhodopsin kinase, whose members are GRK1 and GRK7; the second subfamily is βARK whose members are GRK2 and GRK3, and the last subfamily is GRK-4-like subfamily (GRK4, GRK5, and GRK6) (Pitcher et al., 1998). The GRKs belong to the AGC protein kinase of serine/threonine kinases with the acronyms PKA, PKG,and PKC. Due to the presence of sequence alignments of kinase domains, the members are placed into this subgroup, as the catalytic domain of GRKs is intensely conserved, centrally positioned in the tri-domain structure (Pearce et al., 2010). Rhodopsin kinase is a member of GRK1, found mainly in mammalian retinal rod cells. Rhodopsin kinase phosphorylates light-activated rhodopsin, thereby binding to the arrestin to terminate the light-activated signaling mechanism.
What happens when a GPCR binds to a G protein?
As the name indicates, when an external signaling particle binds to a GPCR, GPCRs communicate with G proteins that are commonly found in the cytosol, thereby triggering the conformational rearrangement within the GPCR. The ensuing conformational change triggers the interaction between the GPCR and the adjacent G protein. G proteins are specific proteins that bind to guanosine triphosphate (GTP) and guanosine diphosphate (GDP) nucleotides. Few G proteins, such as Ras signaling protein, are small proteins having a single subunit, while most G proteins associated with GPCRs are heterotrimeric. G proteins possess three polypeptides structured into two distinct, well-designed units: the α-subunit and the βγ-dimer. From an evolutionary perspective, the GTPase superfamily is conserved throughout evolution. Post-translationally, Gα and Gβγ-subunits are lipidated and predominantly confined at the surface of the inner leaflet of the plasma membrane. As mentioned above, α-subunit can inherently interact with the guanine nucleotide (GDP during the inactive state and GTP when activated). The domain exhibits GTPase activity enabling GTP hydrolysis and, subsequently, G protein deactivation (Cabrera-Vera et al., 2003). In the normal state, G protein exists as a heterotrimeric complex with GDP binding to the α-subunit. As the ligand binds to the receptor, the ensuing events induce a conformational change in the receptor, or binding may arise to stabilize the existing active conformation. The resulting processes cause the association of Gαβγ heterodimer. Heterotrimeric G proteins are also classified according to their Gα-subunit into four main groups (Fig. 1), Gαs, Gαi/o, Gαq/11, and Gα12/13. GPCRs often bind selectively to specific Gα protein family members, although more promiscuous coupling is also seen sometimes (Hermans, 2003). Further details about G proteins will be delivered under the signaling section.
How does GPCR signaling work?
GPCR activation and signaling are regulated by a process called receptor desensitization, which is characterized by the loss of receptor response due to prolonged or overstimulation (Lefkowitz, 1998). GPCR desensitization is described as the physical uncoupling of G proteins from their couple receptors, subsequently resulting in a loss in receptors’ capability to activate and initiate further intracellular signaling (Penela et al., 2003). The receptor desensitization process initiated by GPCR phosphorylation that can be provoked by second messenger kinases, such as PKA and PKC, which can phosphorylate and affect the responsiveness of both agonists occupied and unoccupied receptors, thereby producing heterologous desensitization (Willets et al., 2003, Hausdorff et al., 1990). Receptor phosphorylation can also be mediated by GRKs, seven isoforms of serine/threonine kinases belonging to the AGC kinase family (Homan and Tesmer, 2014), phosphorylating specifically agonist-occupied active GPCRs, mediating the homologous receptor desensitization process (Kelly et al., 2008, Willets et al., 2003) (Fig. 1). Receptor activation provokes GRK recruitment and consequently phosphorylates the receptor’s serine/threonine moieties in the third intracellular loop or C-terminal tail (Lefkowitz, 1993, Benovic et al., 1991), inducing the formation of phosphorylation barcoding, a specific phosphorylation configuration of the receptor (Penela et al., 2010). Receptor phosphorylation subsequently enhances the affinity for β-arrestin protein binding (Scheerer and Sommer, 2017). This physical association between GPCR and β-arrestin protein “phosphorylated GPCR-β-arrestin complexes” further inhibits GPCR and G-protein interaction. Accordingly, the termination of the G protein-dependent signaling cascade commences (Gurevich and Gurevich, 2019). Receptor-arrestin complexes are subjected to clathrin-mediated endocytosis. Depending on the interaction potency, the receptors undergo recycling, internalization into endosomes, or degradation (Penela et al., 2010, Kelly et al., 2008). The GPCRs can be classified according to the strength of the GPCR-β-arrestin interaction into class A GPCR, weak interaction receptors such as β2adrenergic receptors. Thus, the formation of a transient receptor-β-arrestin complex undergoes recycling into the cell membrane. Also, class B GPCR characterized by strong receptor-β-arrestin interaction, such as vasopressin V2receptor, induces sustained internalization into endosomes (Luttrell and Lefkowitz, 2002). Importantly, Thomsen et al. (2016)described the strong interaction between receptors and β-arrestin as supercomplexes or mega complexes mediating sustained/prolonged G protein signaling intracellularly.
What is the secretin family?
Class B GPCRs (Secretin family):Class B GPCRs are acknowledged as the secretin family comprising over 15 receptors for binding peptide hormones. The secretin family constitutes essential drug targets in several human afflictions, namely diabetes (type 2), cardiovascular disease, cancer, headache, neurodegeneration, and psychiatric disorders (Alexander et al., 2013, Pal and Xu, 2012). One of the most important members of class B GPCR is the glucagon-like peptide-1 receptor (GLP1R). GLP1R is mainly expressed in pancreatic β-cells, where it pairs between Gαq and Gαi proteins, regulating the cellular concentration of calcium and inhibiting intracellular cyclic AMP (cAMP respectively). Additionally, it activates adenylyl cyclase (AC) and elevates intracellular cAMP via stimulator G (Gαs) protein, major signaling pathways activating the synthesis and release of insulin and subsequently lowering glucose concentration (Nadkarni and Holz, 2014, Buteau, 2008). Therapies based on GLP1 and GLP1R agonists, such as dipeptidyl peptidase 4 inhibitors, affect glucose metabolism through numerous mechanisms. Glucose homeostasis entirely depends on the complex interaction of several hormones, such as insulin, amylin, gastrointestinal peptides, and glucagon. Aberrant regulation of these hormones may induce clinical manifestations and, finally, diabetes.
What is the function of GPCR?
GPCRs function as communication inboxes like peptides, proteins, lipids, sugars, and light energy. The received messages inform cells about the presence or absence of light-sustaining molecules or nutrients in the immediate surroundings, or they deliver messages sent by other cells. Over 1000 human GPCRs have been discovered, and each works differently regarding the signals they receive (Sommer et al., 2020). The new members’ list is growing daily with advances in new techniques, such as cryogenic electron microscopy and crystallography. The function of new GPCRs and the ligand they activate remain partially comprehended. Research is ongoing yearly to find novel ligands of orphan GPCR (GPCR without ligand is known as orphan GPCR). The GPCR family has four main subfamilies: Class A GPCRs (Rhodopsin-like receptors), Class B GPCRs (Secretin family), Class C GPCRs (Metabotropic Glutamate receptors), and Class F GPCRs (Frizzled and Smoothened receptors) (Fig. 1).
What are the biological functions of living organisms?
Examples of daily biological functions include neuronal communication and neurotransmission in the process of learning and memory, secretion (hormones, sweat, and saliva), muscle contraction, cellular growth, differentiation and migration during wound healing, and immunity to fight infections. Among the different transducers for such life-dependent signals is the large family of G protein-coupled receptors (GPCRs). GPCRs constitute roughly 800 genes, corresponding to 2% of the human genome. While GPCRs control a plethora of pathophysiological disorders, only approximately one-third of GPCR families have been deorphanized and characterized. Recent drug data show that around 40% of the recommended drugs available in the market target mainly GPCRs. In this review, we presented how such system signals, either through G protein or via other players, independent of G protein, function within the biological system. We also discussed drugs in the market or clinical trials targeting mainly GPCRs in various diseases, including cancer.
What are the problems with GPCRs?
Perhaps a greater problem is the stability of purified GPCRs in detergents compatible with crystallography. For example, the β2adrenoceptor (β2AR) and many other GPCRs are not stable in the detergents used to obtain rhodopsin crystals; and thus far rhodopsin crystals have not been obtained in dodecylmaltoside, a detergent in which the β2AR is relatively stable. GPCRs tend to be more stable in non-ionic detergents with relatively long alkyl chains. These detergents may form larger micelles that prevent the formation of crystal contacts [41]. Another problem is the potential for both structural and conformational heterogeneity in GPCRs. By structural heterogeneity I mean heterogeneity in posttranslational modifications such as glycosylation, phosphorylation and palmitoylation. These sources of heterogeneity can often be eliminated by site directed mutagenesis of the protein, or enzymatic removal of sugars and phosphates. This source of heterogeneity is minimized if the GPCR can be expressed in bacteria.
How to obtain 3D crystals?
Three-dimensional crystals have also been obtained from bovine rhodopsin solubilized from rod outer segments using the detergent lauryldimethylamine-oxide (LDAO) and subjected to lectin chromatography followed by detergent exchange into n-octyltetraoxyethylene (C8E4) followed by anion exchange chromatography [26, 28]. This more extensive purification procedure, which would be expected to remove all but tightly bound lipid, resulted in crystals diffracting at 2.6 Å [26]. These crystals have a P31space group and crystal contacts form primarily within the transmembrane domains and the intracellular and extracellular loop structures point into solvent filled cavities. As a result, the loop structures may assume a more native structure in the P31crystal form compared to the P41crystals [29].
Where did rhodopsin come from?
The first structures of rhodopsin came from cryoelectron microsopy of two-dimensional crystals of bovine rhodopsin from Gebhard Schertler’s group [15–19]. While the resolution of these structures was limited (ranging from 5 to 9Å), they provided the first picture of the orientation of the TM segments in a lipid environment. These structures, together with the subsequent computational work from Baldwin [20] provided the template from which most molecular models for other GPCRs were generated.
Which crystal forms are the most divergent?
A comparison of the rhodopsin structures determined from the P41[25] and P31[26] crystal forms. The loop connecting TM5 and TM6 (shown in red) is the most divergent sequence.
What are the major obstacles to obtaining structures of other GPCRs?
The major obstacles to obtaining structures of other GPCRs include protein production and purification, and protein stability and homogeneity . In terms of production, it is now possible to generate sufficient quantities (tens of milligrams) of several GPCRs for crystal screening using bacterial, yeast, insect cell, and mammalian cell expression systems [35–37] [38–40]. The availability of robotic systems for preparing setups of 100 nanoliter volumes (or smaller) has enabled large parameter screens with relatively small amounts of protein. As such, protein production is no longer the major limitation for crystallography efforts.
What are the structural signatures of GPCRs?
GPCRs share a common structural signature of seven hydrophobic transmembrane (TM) segments , with an extracellular amino terminus and an intracellular carboxyl terminus (Figure 1) . GPCRs share the greatest homology within the TM segments. The most variable structures among the family of GPCRs are the carboxyl terminus, the intracellular loop spanning TM5 and TM6, and the amino terminus. The greatest diversity is observed in the amino terminus. This sequence is relatively short (10–50 amino acids) for monoamine and peptide receptors, and much larger (350–600 amino acids) for glycoprotein hormone receptors, and the glutamate family receptors. The largest amino terminal domains are observed in the adhesion family receptors.
Is rhodopsin a stable GPCR?
Rhodopsin is better suited for structural studies than most other GPCRs because it is possible to obtain large quantities of highly enriched protein from bovine retina. Rhodopsin is also a remarkably stable GPCR, retaining function under conditions that denature many other GPCRs.
What is the interaction between GPCRs and their extracellular ligands?
The interaction between GPCRs and their extracellular ligands has proven to be an attractive point of interference for therapeutic agents. For that reason, the pharmaceutical industry has developed biochemical drug discovery assays to investigate these ligand–GPCR interactions, such as scintillation proximity assays (Alouani, 2000) or the less frequently employed fluorescence polarization assays ( Banks and Harvey, 2002; Harris et al., 2003) and fluorescence intensity distribution analysis assays ( Auer et al., 1998; Zemanova et al., 2003). All the aforementioned biochemical binding assays rely on the competition of the test compound with a labeled reference ligand. An obvious disadvantage of these binding assays is the risk of missing noncompetitive, allosteric ligands. Further, the binding assay does not elucidate functional aspects of test compound activity, such as full/partial agonism, neutral antagonism, inverse agonism, or positive modulation. To expand compound testing in this direction, there is a need for functional high‐throughput assays, possibly measuring GPCR activity in a more physiological, cellular background.
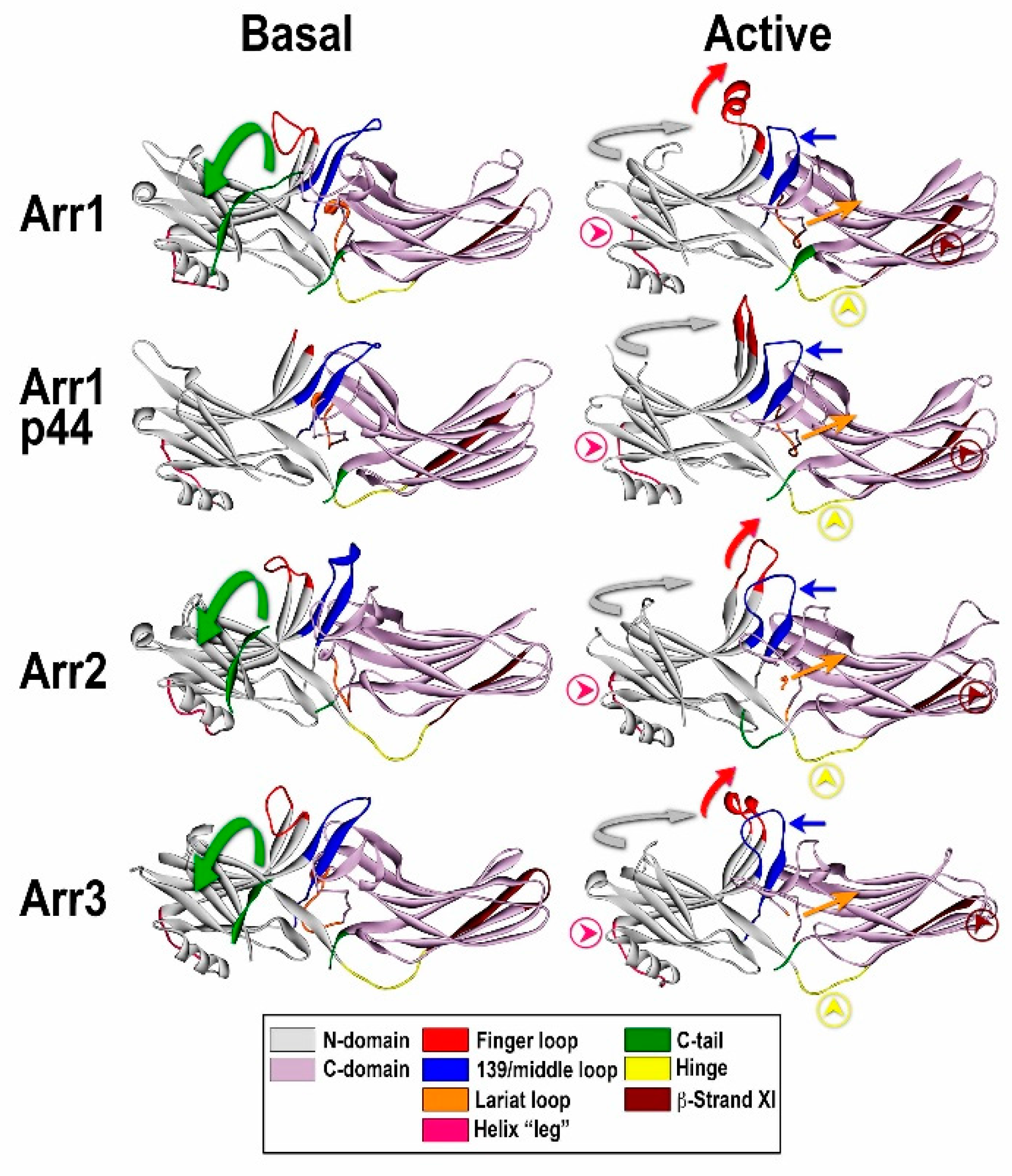
How is arrestin recruited to the plasma membrane?
Scheme of GPCR internalization assays. After agonist stimulation, the GPCR becomes phosphorylated by a GRK on its carboxy‐terminal tail. Arrestin is recruited to the plasma membrane by the GRK‐phosphorylated GPCR. Arrestin then targets the GPCR to clathrin‐coated pits for endocytosis. Depending on the stability of the specific GPCR–arrestin interaction, arrestin is either released after the formation of clathrin‐coated pits or cointernalized with the GPCR‐loaded vesicles. The internalization process may be monitored by a fluorophore label on the agonist, on tN or C terminus of the GPCR, or on the arrestin.
What is the most important class of therapeutic targets?
GPCRs are the most important class of therapeutic targets ( Ma and Zemmel, 2002 ). Approximately 45% of all known pharmaceutical drugs are directed against transmembrane receptors (Drews, 2000), largely against GPCRs. GPCRs are involved in a broad diversity of physiological functions, such as pain perception, chemotaxis, neurotransmission, cardiovascular actions, and metabolism, and finding ways to modulate GPCR signaling remains a major focus of pharmaceutical research.
Why are GPCRs important?
GPCRs are the largest family of membrane proteins in human. Because they regulate almost all physiological processes, GPCRs have always been important targets for drug development. Asthma is one of the most common chronic diseases, and NPSR1, a member of family A GPCRs, shows significant association with asthma.
What are the functions of GPCR?
GPCRs are involved in a broad diversity of physiological functions, such as pain perception, chemotaxis, neurotransmission, cardiovascular actions, and metabolism, and finding ways to modulate GPCR signaling remains a major focus of pharmaceutical research.
How does GPCR activate G-proteins?
In turn, the GPCR can then activate a coupled G-protein by exchanging its bound GDP for a GTP. The G-protein's α subunit, together with the bound GTP, can then dissociate from the β and γ subunits affecting intracellular signaling proteins.
What are the three major classes of GPCR?
GPCR signal transduction mechanisms have been characterized in three major classes: Gq (phospholipase C), Gi, and Gs (inhibition and stimulation of cAMP production, respectively).
Why are GPCRs important?
In addition, acquired mutations in certain GPCRs cause abnormal increases in receptor activity and expression in cell membranes, which can give rise to cancer. Because GPCRs play specific roles in human disease, they have provided useful targets for drug development.
What type of receptor does epinephrine bind to?
Epinephrine binds to a type of G protein-coupled receptor known as a beta-adrenergic receptor. When stimulated by epinephrine, this receptor activates a G protein that subsequently activates production of a molecule called cAMP (cyclic adenosine monophosphate).
What is the GPCR?
A GPCR is made up of a long protein that has three basic regions: an extracellular portion (the N-terminus), an intracellular portion (the C-terminus), and a middle segment containing seven transmembrane domains . Beginning at the N-terminus, this long protein winds up and down through the cell membrane, with the long middle segment traversing ...
What is the G protein?
Alternative Titles: GPCR, heptahelical receptor, seven-transmembrane receptor. G protein-coupled receptor (GPCR), also called seven-transmembrane receptor or heptahelical receptor, protein located in the cell membrane that binds extracellular substances and transmits signals from these substances to an intracellular molecule called a G protein ...
What is the effect of activation of the G protein?
Activation of the G protein initiates a series of intracellular reactions that end ultimately in the generation of some effect, such as increased heart rate in response to epinephrine or changes in vision in response to dim light ( see second messenger ).
What is the last domain of a GPCR?
The last of the seven domains is connected to the C-terminus. When a GPCR binds a ligand (a molecule that possesses an affinity for the receptor ), the ligand triggers a conformational change in the seven-transmembrane region of the receptor. This activates the C-terminus, which then recruits a substance that in turn activates ...
Which drugs stimulate the airway opening in the lungs?
The drugs salmeterol and albuterol, which bind to and activate beta-adrenergic GPCRs, stimulate airway opening in the lungs and thus are used in the treatment of some respiratory conditions, including chronic obstructive pulmonary disease and asthma. Kara Rogers. History at your fingertips.
What is the GEF domain?
The GEF domain may be attached to an inactive -subunit of a heterotrimeric G-protein when the receptor is inactive. These "G-proteins" are a trimer of subunits (known as G, G, and G, respectively) that are rendered inactive when reversibly attached to Guanosine diphosphate (GDP) (or, alternatively, no guanine nucleotide) but active when bound to guanosine triphosphate (GTP). The GEF domain allosterically activates the G-protein by enabling the exchange of a molecule of GDP for GTP at the G—subunit protein's upon receptor activation. The cell maintains a 10:1 cytosolic GTP: GDP ratio to ensure GTP exchange. The G-protein subunits detach from the receptor and from each other at this stage, resulting in a G-GTP monomer and a tightly coupled G dimer that can now control the activity of other intracellular proteins. The palmitoylation of G and the presence of an isoprenoid moiety that has been covalently attached to the C-termini of G, however, limit the amount to which they can spread.
How does a GPCR signal transduction occur?
When an agonist binds to a GPCR, the receptor undergoes a conformational shift that is conveyed to the bound G subunit of the heterotrimeric G protein through protein domain dynamics. The activated G subunit exchanges GTP for GDP, resulting in the G subunit's separation from the G dimer and from the receptor. The fragmented G and G subunits engage with other intracellular proteins to continue the signal transduction cascade, while the released GPCR can rebind to another heterotrimeric G protein to form a new complex ready to start a new round of signal transduction.
How do GPCRs interact with G proteins?
GPCRs interact with G proteins in the plasma membrane, as their name suggests. When a signalling molecule interacts with a GPCR, the GPCR undergoes a conformational shift. The GPCR and a neighbouring G protein then interact as a result of this alteration.
What is the GPCR?
GPCR Full Form: G-protein-coupled receptors (GPCRs) are the biggest and most diversified collection of membrane receptors in eukaryotes. These cell surface receptors act like an inbox for communications in the form of light energy, peptides, lipids, carbohydrates, and proteins. Cells receive these messages to alert them of the presence or absence of life-sustaining light or nutrients in their surroundings, or to relay information from other cells.
What is the role of -arrestins in G-protein coupling?
Once attached, -arrestins sterically impede G-protein coupling and may recruit other proteins, resulting in the formation of signalling complexes that are implicated in the activation of the extracellular signal-regulated kinase (ERK) pathway or receptor endocytosis (internalization). Because these Ser and Thr residues are frequently phosphorylated as a result of GPCR activation, -arr-mediated G-protein dissociation and GPCR internalisation are essential processes of desensitisation. Furthermore, internalised "mega-complexes" containing a single GPCR, -arr (in the tail conformation), and heterotrimeric G protein exist and may be responsible for endosome protein signalling.
What are the functions of G proteins?
When a G protein is active, its GTP-bound alpha subunit and beta-gamma dimer can interact with other membrane proteins involved in signal transduction to relay messages throughout the cell. Different enzymes that make second messengers, as well as certain ion channels that allow ions to behave as second messengers, are specific targets for activated G proteins. Some G proteins enhance these targets' activity, while others inhibit it. The alpha, beta, and gamma subunits of G proteins are encoded by numerous genes in vertebrate genomes. These genes encode a variety of subunits that assemble in a variety of ways to form a varied family of G proteins.
How does protein kinase A regulate cell metabolism?
Protein kinase A regulates cell metabolism by phosphorylating particular committed enzymes in the metabolic pathway, making it a key enzyme in cell metabolism. It can also control the expression of particular genes, cellular secretion, and membrane permeability. Two catalytic and two regulatory subunits make up the protein enzyme. The complex is inactive when there is no cAMP present. When cAMP binds to regulatory subunits, their conformation changes, causing the regulatory subunits to dissociate, activating protein kinase A and allowing for additional biological consequences.
What is the function of G protein-coupled receptors?
G protein-coupled receptors function as cell membrane receptors for the steroid hormone 20-hydroxyecdysone.
Can GPCRs bind 20E?
GPCRs can bind 20E in the cell membrane and after being isolated, suggesting GPCRs as cell membrane receptors of 20E. This review deepens our understanding of GPCRs as steroid hormone cell membrane receptors and the GPCR-mediated signaling pathway of 20E (20E-GPCR pathway), which will promote further study of steroid hormone signaling via GPCRs, ...
Do GPCRs bind steroid hormones?
Recent studies have suggested that GPCRs transmit animal steroid hormone signals. Certain GPCRs have been shown to bind steroid hormones, for example, G protein-coupled estrogen receptor 1 (GPER1) binds estrogen in humans, and Drosophila dopamine/ecdysteroid receptor (DopEcR) binds the molting hormone 20-hydroxyecdysone (20E) in insects.
