What is the law of reciprocal proportions?
Why is the law of reciprocal proportions important? 2 Answers. After defining the law of definite proportions and law of multiple proportions, we could successfully understand how two elements combine together. If two elements combine with a third element, then the ratio of masses of the two elements for unit mass of third element, is a simple ...
What does law of reciprocal proportions mean?
The law of reciprocal proportions states that. If two different elements combine separately with a fixed mass of a third element, the ratio of the masses in which they do so are either the same as or a simple multiple of the ratio of the masses in which they combine with each other.
What does law of equivalent proportions mean?
law of equivalent proportions- (chemistry) law stating that the proportions in which two elements separately combine with a third element are also the proportions in which they combine together law of reciprocal proportions law of nature, law- a generalization that describes recurring facts or events in nature; "the laws of thermodynamics"
Who discovered the law of multiple proportions?
The Law of Multiple Proportions is defined as: “if two elements combine to form more than one compound, the mass ratios of the second element that combine with a fixed mass of the first element will always be ratios of minuscule whole numbers.”. This law, sometimes known as Dalton's Law or Dalton's Law of Multiple Proportions, was proposed ...
What is law of multiple proportions explain?
In chemistry, the law of multiple proportions states that if two elements form more than one compound, then the ratios of the masses of the second element which combine with a fixed mass of the first element will always be ratios of small whole numbers.
Who gave law of multiple proportions Class 11?
chemist John DaltonThis law of multiple proportions is also known as Dalton's law, because this law was announced (1803) by the English chemist John Dalton.
What is the law of multiple proportion give an example?
In terms of masses also, every 2.016 g of hydrogen ( g.at. mass of hydrogen is 1.008g) combines with 15.999 g of oxygen to form water and 31.998g of oxygen to form hydrogen peroxide. Hence the ratio is 15.999 : 31.998 or 1:2 which is a simple whole number ratio.
Who proposed law of proportion?
chemist Joseph Louis ProustThe law of definite proportions was first put forward by the French chemist Joseph Louis Proust in the year 1779.
How did John Dalton discover the law of multiple proportions?
Dalton's measurements, crude as they were, allowed him to formulate the Law of Multiple Proportions: When two elements form more than one compound, the masses of one element that combine with a fixed mass of the other are in a ratio of small whole numbers.
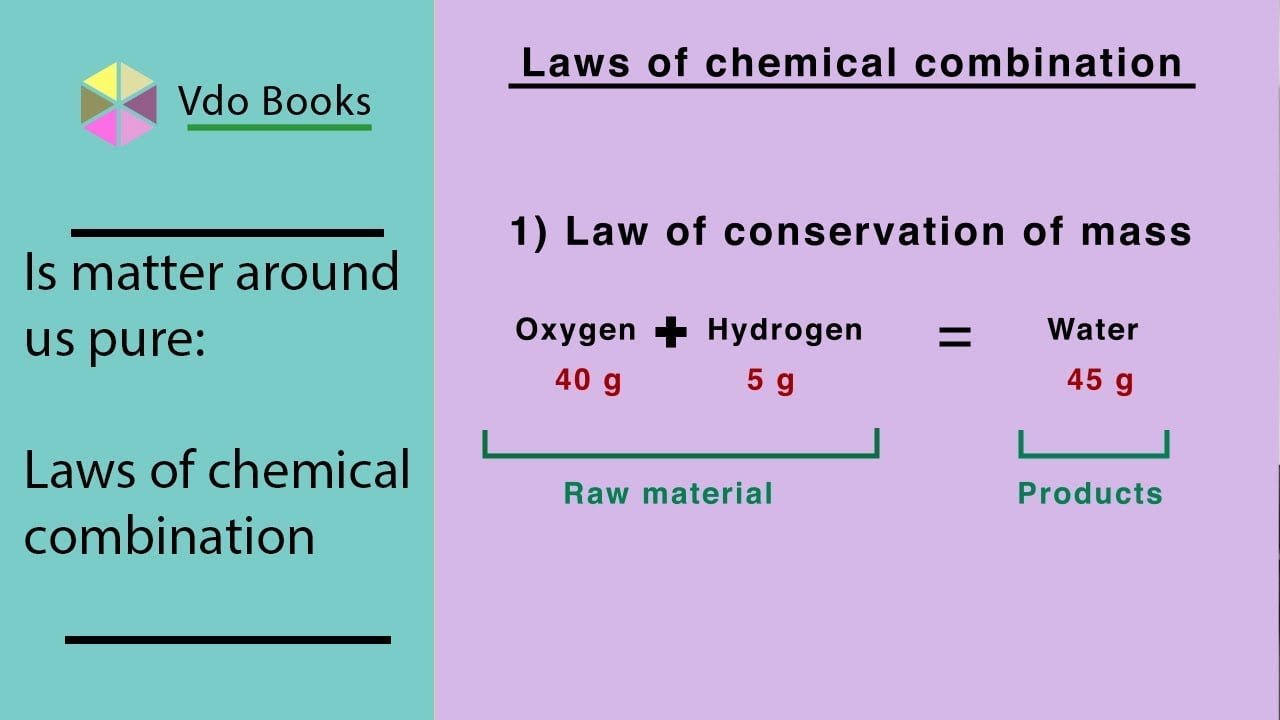
Who gave law of conservation of mass?
Antoine Lavoisier'sThe Law of Conservation of Mass dates from Antoine Lavoisier's 1789 discovery that mass is neither created nor destroyed in chemical reactions.
When was given the law of definite proportion?
History. The law of constant proportion was given by Joseph Proust in 1797. This observation was first made by the English theologian and chemist Joseph Priestley, and Antoine Lavoisier, a French nobleman and chemist centered on the process of combustion.
What is law of definite proportion Class 11?
law of definite proportion states that in a given compound elements are combined in a fixed ratio by mass.
Who gave law of reciprocal proportion?
Jeremias RitcherThe law of reciprocal proportions was proposed by Jeremias Ritcher in 1792. It states that, "If two different elements combine separately with the same weight of a third element, the ratio of the masses in which they do so are either the same or a simple multiple of the mass ratio in which they combine."
What is the law of definite proportions Class 11?
law of definite proportions, statement that every chemical compound contains fixed and constant proportions (by mass) of its constituent elements.
Who gave law of reciprocal proportion?
Jeremias RitcherThe law of reciprocal proportions was proposed by Jeremias Ritcher in 1792. It states that, "If two different elements combine separately with the same weight of a third element, the ratio of the masses in which they do so are either the same or a simple multiple of the mass ratio in which they combine."
What is Dalton atomic theory class 11?
(i) All matter is made of very tiny particles called atoms. (ii) Atoms are indivisible particles, which cannot be created or destroyed in a chemical reaction. (iii) Atoms of a given element are identical in mass and chemical properties. (iv) Atoms of different elements have different masses and chemical properties.
What is law of conservation of mass Class 11?
The law of conservation of mass states that mass in an isolated system is neither created nor destroyed by chemical reactions or physical transformations. According to the law, the mass of the products in a chemical reaction must equal the mass of the reactants.
1. What are the limitations of the Law of Multiple Proportions?
The law of multiple proportions is demonstrated best using simple compounds. For example, if anyone tried to demonstrate the same using the hydroca...
2. What is the difference between the Law of Definite Proportions and the Law of Multiple Proportion...
The law of definite proportions asserts that samples of a compound will always contain the same mass proportion of constituents, as opposed to the...
3. Give two examples of the Law of Multiple Proportions.
The examples are as follows:Example 1: Carbon (C) and Oxygen (O) combine to form the compounds CO and \[CO_{2}\].CO2 contains 12g of carbon and 16g...
4. List the shortcomings of Dalton’s atomic theory.
Some important demerits of Dalton’s atomic theory are listed below.The presence of subatomic particles was not accounted for in the theory (it sugg...
5. Is it true that electrons exist?
As most of us know, the neutron is a negatively charged particle that orbits the nucleus of an atom of matter. At any given time, no two electrons...
6. How can CBSE Class 11 students obtain free Law of Multiple Proportions questions and answers from...
Students should be aware that the Class 12 Chemistry syllabus is extensive, and that to receive good grades, they must answer all of the important...
7. Give the Importance of Atomic Theory?
Knowing the atomic theory allows us to:know the origin, predict the chemical properties and physical properties of the atomknow the atomic structur...
8. Give the Most Accepted Atomic Structure?
There is an important point about the Bohr model, which is no longer accepted in the current models of the atom. In this Bohr model, still, the ele...
9. What is the Structure of an Atom?
The matter has mass and takes up space. Atoms are defined as basic building blocks of matter, and they cannot be chemically subdivided by ordinary...
What is the proportion of an element?
Proportions: Proportions are the amounts of atoms that make up an element.
When two elements combine in different ratios to form different compounds, the mass of the element combining with the fixed mass?
This shows that the law of multiple proportions is followed.
What happens when two elements combine to form more than one compound?
The law of multiple proportions, states that when two elements combine to form more than one compound, the mass of one element, which combines with a fixed mass of the other element, will always be ratios of whole numbers.
Is the whole number ratio of hydrogen in methane (CH4) to hydrogen in methane (CH4)?
False, because the correct statement is: The whole number ratio of hydrogen in Methylene (CH2) to hydrogen in methane (CH4) is equal to 2:4.
Does the law of multiple proportions apply to SO2 and CO2?
Let us remember that the law of multiple proportions only applies to compounds composed of the same elements. It does not apply to, let's say, SO2 and CO2 because one compound has sulfur (S) and one has carbon (C).
What is the law of multiple proportions?
Law of multiple proportions, statement that when two elements combine with each other to form more than one compound, the weights of one element that combine with a fixed weight of the other are in a ratio of small whole numbers. For example, there are five distinct oxides of nitrogen, and the weights of oxygen in combination with 14 grams ...
Who was the first to announce the law of multiple proportions?
The law was announced (1803) by the English chemist John Dalton, and its confirmation for a wide range of compounds served as the most powerful argument in support of Dalton’s theory that matter consists of indivisible atoms. Read More on This Topic. chemical bonding: The law of multiple proportions. The second step toward Dalton’s synthesis was ...
What was the second step in Dalton's synthesis?
The second step toward Dalton’s synthesis was the recognition of the existence of related series of compounds formed by the same elements. It was established, for example, that, whereas 28 grams of carbon monoxide invariably consists of 12 grams of carbon…
What is the law of multiple proportions?
Jump to navigation Jump to search. In chemistry, the law of multiple proportions states that if two elements form more than one compound, then the ratios of the masses of the second element which combine with a fixed mass of the first element will always be ratios ...
Who first proposed the law of definite proportions?
John Dalton first expressed this observation in 1804. A few years previously, the French chemist Joseph Proust had proposed the law of definite proportions, which expressed that the elements combined to form compounds in certain well-defined proportions, rather than mixing in just any proportion; and Antoine Lavoisier proved the law ...
Does the law work with non-stoichiometric compounds?
The law fails with non-stoichiometric compounds and also doesn't work well with polymers and oligomers .
Did Proust find the law of multiple proportions?
Scholars who have reviewed the writings of Proust found that he had enough data to have discovered the law of multiple proportions himself, but somehow he did not. With regards to the aforementioned tin oxides, had Proust adjusted his figures for a tin content of 100 parts for both oxides, he would have noticed that 100 parts ...
Who gave the law of multiple proportions?
In this article, we shall study the law of multiple proportions. The law of multiple proportions was given by British scientist John Dalton in 1803.
What is the ratio of different masses of oxygen combining with fixed mass of carbon (1 g)?
From statements (1) and (2), the ratio of different masses of oxygen combining with fixed mass of carbon (1 g) is 1.33 : 2.66 i.e. 1 : 2, which is simple whole number ratio. Thus, the data illustrate the law of multiple proportions.
What is the ratio of carbon and oxygen?
Carbon and oxygen combine together to give two compounds carbon dioxide (CO 2) and carbon monoxide (CO) Thus the ratio of different weights of oxygen (32 and 16) combining with a fixed weight of carbon (12) is 32 : 16 i.e. 2 :1, which is simple whole number ratio.
Law of multiple proportions
When two elements combine with each other to form two or more compounds, the ratios of the masses of one element that combines with the fixed ratio of the other are simple whole numbers.
Numerical on multiple proportions
For carbon dioxide, 2.66g of oxygen/1.00g of carbon = 2.66. For carbon monoxide, 1.33 g of oxygen/1.00 g of carbon = 1.33. The two ratio are in the proportion 2.66/1.33 = 2:1.There for the ratio of masses of oxygen that combine with the same mass of carbon is 2:1 i.e. a simple ratio.
What are the two elements that make up the law of multiple proportions?
This is a worked example of a chemistry problem using the law of multiple proportions. Two different compounds are formed by the elements carbon and oxygen . The first compound contains 42.9% by mass carbon and 57.1% by mass oxygen. The second compound contains 27.3% by mass carbon and 72.7% by mass oxygen.
Which postulate states that the mass of one element that is a fixed mass of the second element is in a?
Solution. The law of multiple proportions is the third postulate of Dalton's atomic theory. It states that the masses of one element which combine with a fixed mass of the second element are in a ratio of whole numbers. Therefore, the masses of oxygen in the two compounds that combine with a fixed mass of carbon should be in a whole number ratio.
Is ratio always obvious?
The ratio won't always be obvious. It takes practice to recognize ratios. In the real world, the law of multiple proportions doesn't always hold. The bonds formed between atoms are more complex than what you learn about in a chemistry 101 class. Sometimes whole number ratios don't apply.
About This Quiz & Worksheet
Some of the questions on the quiz will provide you with data on different compounds. You will then be asked if the scenario presented demonstrates the law of multiple proportions. Other questions are math problems asking you to identify the whole number ratio from the given data.
Additional Learning
Once you have finished your quiz, make sure to peruse the partner lesson called Law of Multiple Proportions: Definition & Examples. This specially designed lesson covers the following objectives: