What kind of receptor does insulin use?
The insulin receptor ( IR) is a transmembrane receptor that is activated by insulin, IGF-I, IGF-II and belongs to the large class of receptor tyrosine kinase.
What are the normal levels of insulin?
Normal insulin levels for non-diabetics are usually between 60 and 100 mg/dl and may rise to 140 mg/dl after eating, reports the University of California. Diabetics may have insulin levels that range from as low as 25 mlU/L at fasting to as high as 30-230 mlU/L after recent glucose administration, reports Medscape.
What does receptor, insulin mean?
What does an insulin receptor do? Insulin receptors are areas on the outer part of a cell that allow insulin in the blood to join or bind with the cell. When the cell and insulin. bind together, the cell can take glucose from the blood and use it for energy.
What is the pharmacological effect of insulin?
Within the liver, insulin stimulates hepatic glycogen synthesis. Insulin promotes hepatic synthesis of fatty acids, which are released into the circulation as lipoproteins. Skeletal muscle effects of insulin include increased protein synthesis and increased glycogen synthesis.
What type are insulin receptors?
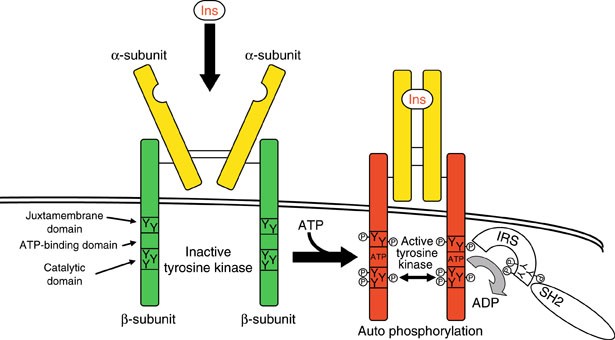
The Insulin Receptor is a type of tyrosine kinase receptor, in which the binding of an agonistic ligand triggers autophosphorylation of the tyrosine residues, with each subunit phosphorylating its partner.
Is insulin a protein receptor?
The receptor for insulin is a large protein that binds to insulin and passes its message into the cell. It has several functional parts. Two copies of the protein chains come together on the outside of the cell to form the receptor site that binds to insulin.
Is insulin receptor a transmembrane receptor?
Abstract. The insulin receptor (IR) is a (αβ)2-type transmembrane tyrosine kinase that plays a central role in cell metabolism.
Which receptors does insulin bind to?
At the cellular level, insulin binds to the insulin receptor (IR) on the plasma membrane (PM) and triggers the activation of signaling cascades to regulate metabolism and cell growth.
Where in the cell is the insulin receptor?
Insulin Receptors are areas on the outer part of a cell that allow the cell to join or bind with insulin that is in the blood. When the cell and insulin bind together, the cell can take glucose (sugar) from the blood and use it for energy. Phe 25B is the active site of insulin.
How does insulin work receptor?
Insulin binds outside the cell to the extracellular domain of its receptor and induces a structural change that is propagated across the membrane to the intracellular kinase domains inside the cell, causing them to activate each other, thus initiating signaling cascades.
Is insulin a second messenger?
In order to explain how insulin regulates a wide variety of biologic functions both on the surface of the cell as well as in its interior, it has been postulated that insulin generates a second messenger at the cell surface.
What type of receptor is the growth hormone receptor?
Type I cytokine receptor familyStructure. Growth hormone receptor (GHR) is a transmembrane protein consisting of 620 amino acids. The receptor is part of the Type I cytokine receptor family of receptors. GHR exists in two forms as a full length membrane-bound receptor and as a soluble GH binding protein (GHBP).
Where are the receptors for insulin located quizlet?
The insulin receptor has two alpha units on the outside of the cell membrane and two beta units that extend from the membrane to the inside of the cell. The two alpha units allow insulin to bind to the insulin receptor, and the beta chains contain tyrosine protein kinase domains.
Is insulin an enzyme?
No, insulin is not an enzyme. Moreover, insulin is a hormone that is created by the pancreas in order to control the amount of glucose present in the bloodstream at any point in time. This hormone is also known to help store glucose in the liver, muscles, and fat.
How many insulin receptors are in a cell?
Scatchard analysis of binding were biphasic and showed high affinity sites with a Kd of about 1.5 nM and capacity of about 10,000 receptors per cell; low affinity sites were much more numerous with a Kd of 88 nM for mouse and 998 nM for rat.
What is the insulin receptor?
The insulin receptor is a member of the ligand-activated receptor and tyrosine kinase family of transmembrane signaling proteins that collectively are fundamentally important regulators of cell differentiation, growth, and metabolism. The insulin receptor has a number of unique physiological and bio ….
What is the initial response to the ligand?
The initial response to the ligand is receptor autophosphorylation for all receptor tyrosine kinases. In most cases, this results in receptor association of effector molecules that have unique recognition domains for phosphotyrosine residues and whose binding to these results in a biological response. For the insulin receptor, this does not occur;
Does insulin phosphorylate substrate proteins?
For the insulin receptor, this does not occur; rather, it phosphorylates a large substrate protein that, in turn, engages effector molecules. Possible reasons for these differences are discussed in this review.
Histologic Distribution Of Insulin And Glucagon Receptors
M. Watanabe, H. Hayasaki, T. Tamayama and M. Shimada Department of Anatomy, Osaka Medical College, Takatsuki, Osaka, Japan Insulin and glucagon are the hormonal polypeptides secreted by the B and A cells of the endocrine pancreas, respectively.
Insulin Receptor
The cellular receptor for insulin helps control the utilization of glucose by cells Cells throughout the body are fueled largely by glucose that is delivered through the bloodstream. A complex signaling system is used to control the process, ensuring that glucose is delivered when needed and stored when there is a surplus.
Insulin Receptors
Insulin Receptors are areas on the outer part of a cell that allow the cell to join or bind with insulin that is in the blood. When the cell and insulin bind together, the cell can take glucose (sugar) from the blood and use it for energy. Phe 25B is the active site of insulin.
The Insulin Receptor And Its Signal Transduction Network
Go to: ABSTRACT Insulin is an anabolic peptide hormone secreted by the b cells of the pancreas acting through a receptor located in the membrane of target cells - major ones being liver (where it promotes glucose storage into glycogen and decreases glucose output), as well as skeletal muscle and fat (where it stimulates glucose transport through translocation of GLUT4), but also b cells, brain cells and in fact most cells, where it has pleiotropic effects.
Insulin Receptor
The insulin receptor (IR) is a transmembrane receptor that is activated by insulin, IGF-I, IGF-II and belongs to the large class of tyrosine kinase receptors.
Insulin Binding And Activation Of The Insulin Receptor
The insulin receptor (IR) is a large, disulphide-linked, glycoprotein that spans the cell membrane with its insulin binding surfaces on the outside of the cell and its tyrosine kinase domains on the inside.
Insulin Receptor Signaling In Normal And Insulin-resistant States
Figure 1. Insulin- and IGF-1-signaling pathways. Activation of insulin and IGF-1 receptors by their ligands initiates a cascade of phosphorylation events.
Where is the insulin receptor located?
Insulin is an anabolic peptide hormone secreted by the b cells of the pancreas acting through a receptor located in the membrane of target cells - major ones being liver (where it promotes glucose storage into glycogen and decreases glucose output), ...
How are insulin receptors synthesized?
The receptors are synthesized as single chain preproreceptors that are processed by a furin-like proteolytic enzyme, glycosylated, folded and dimerized to yield the mature a2b2receptor. In cells expressing both insulin and IGF-I receptors, hybrid receptors are formed consisting of one half of each (31).
How many exons are in the insulin receptor?
The insulin receptor has a modular structure (for review see ref. 25) encoded by a gene (located on chromosome 19) with 22 exons and 21 introns (26, Fig. 3). The short exon 11 that encodes a 12-amino acid sequence is alternatively spliced, resulting in two receptor isoforms (A and B) that differ slightly in affinity for insulin (27-29). The B isoform binds the IGFs with at least 100 times lower affinity than insulin, while the A isoform has significantly higher affinity than the B isoform for IGF-I and especially IGF-II (30) and may play a role in tumorigenesis. The IGF-I receptor binds IGF-II with a lower affinity than IGF-I and insulin with a 500-fold lower affinity. The receptors are synthesized as single chain preproreceptors that are processed by a furin-like proteolytic enzyme, glycosylated, folded and dimerized to yield the mature a 2 b 2 receptor. In cells expressing both insulin and IGF-I receptors, hybrid receptors are formed consisting of one half of each (31). Their physiological role is unknown. Comparative sequence analysis of the insulin/IGF-I receptors and the related EGF receptor (32) had led Bajaj et al. to suggest (Fig. 3) that the N-terminal half consists of two large homologous globular domains, L1 and L2, separated by a cysteine-rich region later predicted to consist of a series of disulfide-linked modules similar to those found in the tumor-necrosis factor (TNF) receptor and laminin. The C-terminal half of the receptors was predicted to consist of three fibronectin type III (FnIII) domains. The second FnIII domain contains a large insert domain (120 residues) of unknown structure containing the site of cleavage between a- and b-subunits. The disulfide bond between each a- and b- subunit involves the cysteins C647 and C860. In addition there are a-a disulfide bonds at C524 in the FnIII-1 domains and between the triplet C682-C683 and C685 in the insert domain (Fig. 3). The intracellular portion of the a-subunit contains the kinase domain flanked by two regulatory regions, a juxtamembrane region involved in docking insulin receptor substrates (IRS) 1-4 and Shc as well as in receptor internalization, and a C-terminal tail. The IGF-I receptor has a similar modular organization (33). The recent progress in the X-ray crystallographic structures of whole ectodomains or fragments of the insulin and IGF-I receptors (see below) has largely validated the structural predictions shown in Fig. 3.
What do the circles on the insulin receptors represent?
The circles marked S1 and S2 symbolize the two insulin receptor binding sites , in a symmetrical antiparallel disposition. The insulin molecule is symbolized by a black dot. a1 and a2: association rate constants for sites 1 and 2 respectively. d1 and d2: dissociation rate constants for sites 1 and 2 respectively. kcr: crosslinking constant. see text for explanations. From reference 64, used with permission.
How does insulin work?
The concept that insulin acts by promoting glucose transport across the membrane of target cells (rather than acting directly on enzymes of intermediary metabolism of glucose) was established in 1949 by the iconic experiment of Rachmiel Levine and colleagues (8), who showed that insulin markedly increased the volume of distribution of non-metabolisable galactose in eviscerated nephrectomized dogs from 45-47% of body weight to 75%, a figure close to that of total body water. From this finding they proposed the following working hypothesis: "Insulin acts upon the cell membrane of certain tissues (skeletal muscle, etc.) in such a manner that the transfer of hexoses (and perhaps other substances) from the extracellular fluid into the cell is facilitated. The intracellular fate of the hexoses depends upon the availability of metabolic systems for their transformation. In the case of glucose, dissimilation, glycogen storage, and transformation to fat are secondarily stimulated by the rapidity of its entry into the cell".
What are the pleiotropic actions of insulin?
Insulin through its receptor affects multiple physiological processes in the organism (left) by increasing (green arrows) or decreasing (red arrows) various intracellular metabolic pathways (right). Inspired by figure 2-1 of reference 1.
Where is insulin secreted?
ABSTRACT. Insulin is an anabolic peptide hormone secreted by the b cells of the pancreas acting through a receptor located in the membrane of target cells - major ones being liver (where it promotes glucose storage into glycogen and decreases glucose output), as well as skeletal muscle and fat ...
