How does acid rain affect plants and animals?
Effects of Acid Rain on Plants and Trees. Dead or dying trees are a common sight in areas effected by acid rain. Acid rain leaches aluminum from the soil. That aluminum may be harmful to plants as well as animals. Acid rain also removes minerals and nutrients from the soil that trees need to grow.
Why is rain so acidic?
However, when rain reacts with certain air pollutants, such as sulfur or nitrogen oxides, the water vapor converts into very diluted forms of sulfuric or nitric acids. The acidity of this rain is on par with that of grapefruit juice, which may not seem like much, but is much more caustic than plain water.
What is the USGS doing about acid rain?
The U.S. Geological Survey (USGS) has been actively studying acid rain for the past 15 years. When scientists learned that acid rain could harm fish, fear of damage to our natural environment from acid rain concerned the American public.
What is acid precipitation and how does it work?
For you to understand acid precipitation, you need to know the meanings of both an acid and precipitation. An acid is measured by the concentration of hydrogen ions and is ranked on what we call the pH scale. Acids have a low value on the pH scale. For example, lemon juice and soft drinks are both very acidic.
Why is acid precipitation important?
The acidic clouds and fog strip important nutrients from their leaves and needles. This loss of nutrients makes it easier for infections, insects, and cold weather to damage trees and forests. Without pollution or acid rain, most lakes and streams would have a pH level near 6.5.
What is acid precipitation and why is this significant?
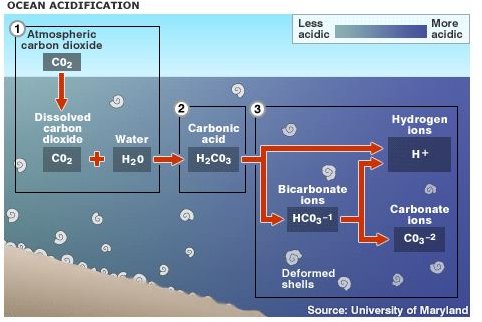
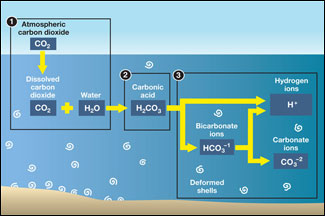
Acid rain results when sulfur dioxide (SO2) and nitrogen oxides (NOX) are emitted into the atmosphere and transported by wind and air currents. The SO2 and NOX react with water, oxygen and other chemicals to form sulfuric and nitric acids. These then mix with water and other materials before falling to the ground.
How can acid precipitation affect organisms?
Acid rain can cause serious problems for many different animals and plants. As a result, the entire food web is affected. For example, acid rain can cause phytoplankton in lakes to die. Insects, which rely on phytoplankton for food, now have less food to eat, and they begin to die as a result.
How does Acid Precipitation affect land and water organisms?
Reduced pH Level in Water Acid rain can make the water in lakes and streams more acidic and discharge toxic amounts of aluminum into a water system. Many aquatic animals cannot thrive in a low pH environment; acid rain has many negative effects on plants and animals in the environment.
Why is acid precipitation such a concern in most countries?
Acid rain produces the premature death of fish living in these waters or may cause changes in the life cycles, in terms of spawning and reproduction of fish species. Acid rain also has an adverse effect on arable soils since it changes the chemistry of the soil and renders it less efficient for agriculture.
What are the effects of acid rain on animals and plants?
Acid rain can also change the composition of soil and bodies of water, making them uninhabitable for local animals and plants. For example, healthy lakes have a pH of 6.5 or higher. As acid rain raises the level of acidity, fish tend to die off. Most fish species can't survive a water pH of below 5.
What effects does acid rain has on plants survival and growth?
Acid rain removes minerals and nutrients from the soil, which are essential for plant growth. Drops of acid rains suck out the nutrients from the plant leaves. Nutrient-fewer leaves become unable to absorb sufficient sunlight. It also affects the photosynthesis process.
How does acid rain affect non living things?
Due to the corrosive nature of the acids it damages both non-living things as well as living organisms. Acid rain poisons rivers and lakes. Fish and other animals cannot live in acid water. It is also bad for buildings as the acid damages calcium carbonate stone.
How does acid rain affect water quality?
Acid rain makes such waters more acidic, which results in more aluminum absorption from soil, which is carried into lakes and streams. That combination makes waters toxic to crayfish, clams, fish, and other aquatic animals. (Learn more about the effects of water pollution.)
How does acid rain affect fish and other aquatic organisms?
Acid rain adversely affects the aquatic ecosystem. It is harmful to aquatic ecosystems because it alters their pH levels and reduces the amount of oxygen available. When the water becomes more acidic, it kills various species of fish and other aquatic lifeforms in these bodies of water.
How is acid rain a threat to biodiversity?
Acid rain causes a cascade of effects that harm or kill individual fish, reduce population numbers, completely eliminate species from a lake, and decrease biodiversity. As acid rain flows through soils in a watershed, it releases aluminum from soils into the lakes and streams located in that watershed.
Which is a cause of acid precipitation?
Acid rain is caused by a chemical reaction that begins when compounds such as sulphur dioxide and oxides of nitrogen are released into the air. These substances can rise very high up into the atmosphere, where they mix and react with water, oxygen, and other chemicals to form more acidic pollutants called acid rain.
Where is acid precipitation most commonly found?
Acid rain is responsible for severe environmental destruction across the world and occurs most commonly in the North Eastern United States, Eastern Europe and increasingly in parts of China and India.
What's the difference between acid rain and acid precipitation?
Acid rain contains acidic substances, which are dispersed in the atmosphere. Other than rain, acid precipitation involves sleet, snow, fog, and cloud vapor. Acid rain is limited to a period of the year, where various types of acid precipitation take place throughout the year.
What are the effects of acid rain on plant growth?
Effects of Acid Rain on Plants and Trees Acid rain also removes minerals and nutrients from the soil that trees need to grow. At high elevations, acidic fog and clouds might strip nutrients from trees' foliage, leaving them with brown or dead leaves and needles.
What is USGS doing about acid rain?
The USGS has been at the forefront of studying the impacts of acid rain for decades. How does acid rain form? What does it do to the landscape? Can it burn you like battery acid? Keep reading to find out more...
How does acid rain affect the environment?
IMPACT OF ACID RAIN ON FORESTS. Acid rain can dissolve certain more soluble elements from the soil, like aluminum. The dissolved aluminum begins to accumulate and can reach toxic levels as it enters local streams and wetlands. Acid rain also removes important nutrients from the soil, such as calcium, potassium, and magnesium.
What is the National Acid Precipitation Assessment Program?
The National Acid Precipitation Assessment Program (NAPAP), a Federal program involving representatives from more than a dozen Federal agencies, has sponsored studies on how acid rain forms and how it affects lakes, crops, forests, and materials.
What causes acid rain?
The main sources of pollutants that trigger acid rain are vehicles and industrial and power-generating plants. The areas of greatest acidity are in the northeastern United States.
What is acid rain?
Acid rain is the term commonly used by scientists to describe rain that is abnormally acidic. What does that mean? Well, plain distilled water, like that used in laboratories, is neutral (not acidic or basic). Since rain naturally has things dissolved in it, it will always be slightly acidic.
Why are historic buildings affected by acid rain?
Because buildings and monuments cannot adapt to changes in the environment, as plants and animals can, historic structures may be particularly affected by acid precipitation. Scientists are studying effective control technologies to limit the emissions from power plants and automobiles that cause acid rain.
Why are forests more susceptible to pests, disease, and injury from freezing and drought?
Lastly, the combination of reduced calcium and excessive aluminum can make forests more susceptible to pests, disease, and injury from freezing and drought, as a proper balance of these nutrients is vital to forest health. A forest of dead trees damaged by acid rain (Credit: Pixabay).
How does acid rain affect ecosystems?
The Effects of Acid Rain on Ecosystems. This figure illustrates the pH level at which key organisms may be lost as their environment becomes more acidic. Not all fish, shellfish, or the insects that they eat can tolerate the same amount of acid. An ecosystem is a community of plants, animals and other organisms along with their environment ...
Why are trees dying in acid rain?
Dead or dying trees are a common sight in areas effected by acid rain. Acid rain leaches aluminum from the soil. That aluminum may be harmful to plants as well as animals. Acid rain also removes minerals and nutrients from the soil that trees need to grow.
What is episodic acidification?
Episodic Acidification. Melting snow and heavy rain downpours can result in what is known as episodic acidification. Lakes that do not normally have a high level of acidity may temporarily experience effects of acid rain when the melting snow or downpour brings greater amounts of acidic deposition and the soil can’t buffer it.
What happens when acidic particles corrode metal?
The acidic particles corrode metal and cause paint and stone to deteriorate more quickly. They also dirty the surfaces of buildings and other structures such as monuments. The consequences of this damage can be costly: loss of detail on stone and metal statues, monuments and tombstones.
Why are my trees dying at high elevations?
At high elevations, acidic fog and clouds might strip nutrients from trees’ foliage, leaving them with brown or dead leaves and needles. The trees are then less able to absorb sunlight, which makes them weak and less able to withstand freezing temperatures.
Does acid rain cause pollution?
Nitrogen Pollution. It’s not just the acidity of acid rain that can cause problems. Acid rain also contains nitrogen, and this can have an impact on some ecosystems. For example, nitrogen pollution in our coastal waters is partially responsible for declining fish and shellfish populations in some areas.
Can plants survive acidic water?
Some types of plants and animals are able to tolerate acidic waters and moderate amounts of alu minum. Others, however, are acid-sensitive and will be lost as the pH declines. Generally, the young of most species are more sensitive to environmental conditions than adults. At pH 5, most fish eggs cannot hatch. At lower pH levels, some adult fish die. Some acidic lakes have no fish. Even if a species of fish or animal can tolerate moderately acidic water, the animals or plants it eats might not. For example, frogs have a critical pH around 4, but the mayflies they eat are more sensitive and may not survive pH below 5.5.